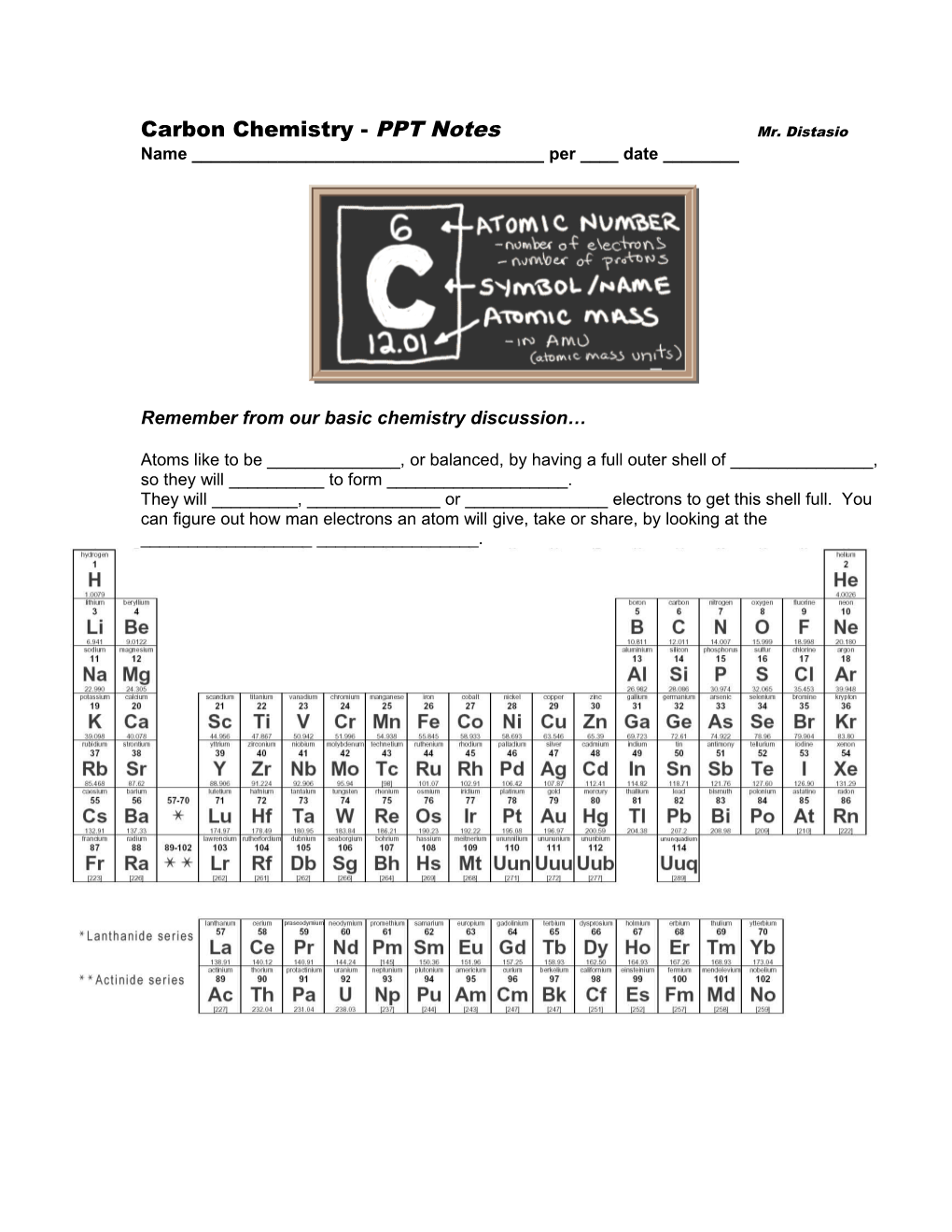
Carbon Chemistry - PPT Notes Mr. Distasio Name ______per ____ date ______
Remember from our basic chemistry discussion…
Atoms like to be ______, or balanced, by having a full outer shell of ______, so they will ______to form ______. They will ______, ______or ______electrons to get this shell full. You can figure out how man electrons an atom will give, take or share, by looking at the ______. Carbon is a particularly important element. ______is based on molecules made from carbon. You are made of ______. Molecules based on carbon are called ______
Carbon is special because it makes ______bonds, and it likes to ______to get the electrons it wants in its outer shell, which means the bonds are ______.
No other organic molecule makes four bonds. ______
Carbon has an atomic number of six. That means it has ___ protons.
It also has six n______so it has an atomic mass of _____.
Six protons means that it has ___ electrons.
It has f______electrons in its outer shell, so it s______four electrons to form partnerships.
______
So, carbon, with four outer shell electrons, tends to ______four electrons to become stable.
Often, it binds with four hydrogens to form ______.
A two carbon chain: ______
______has three carbon atoms. ______has four. How many carbon atoms would be in a chain in pentane? ____ How many hydrogen atoms? ___ How many carbon atoms would a hexane molecule have? ____ How many hydrogen atoms? ______How many carbon atoms would an octane molecule in gasoline have? ____ How many hydrogens? ___ Carbon likes to bond with other ______atoms to make carbon ______. Some chains are short, while others are long. Some chains are ______, while others ______, like a tree.
When wet, carbon atoms bind with one another to form ______structures.
In living things, carbon often bonds to ______, ______, and ______.
______
Carbon makes up the basis of four basic ______that form all living things:
______
______
______
______