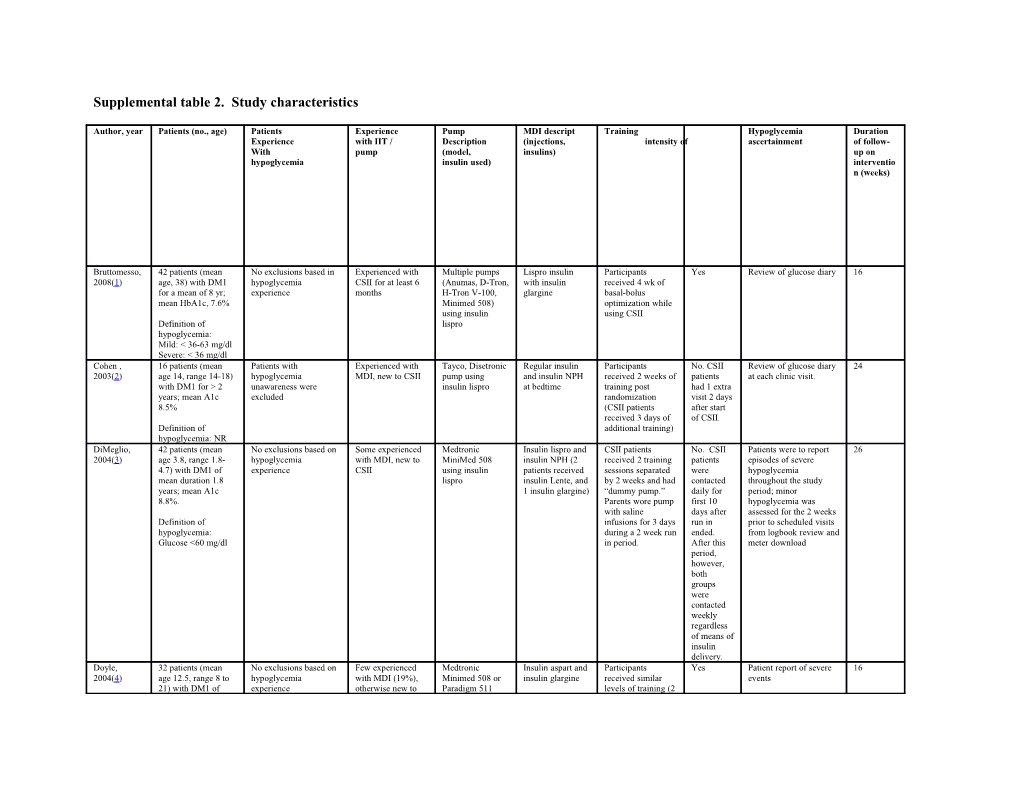
Supplemental table 2. Study characteristics
Author, year Patients (no., age) Patients Experience Pump MDI descript Training Hypoglycemia Duration Experience with IIT / Description (injections, intensity of ascertainment of follow- With pump (model, insulins) up on hypoglycemia insulin used) interventio n (weeks)
Bruttomesso, 42 patients (mean No exclusions based in Experienced with Multiple pumps Lispro insulin Participants Yes Review of glucose diary 16 2008(1) age, 38) with DM1 hypoglycemia CSII for at least 6 (Anumas, D-Tron, with insulin received 4 wk of for a mean of 8 yr; experience months H-Tron V-100, glargine basal-bolus mean HbA1c, 7.6% Minimed 508) optimization while using insulin using CSII Definition of lispro hypoglycemia: Mild: < 36-63 mg/dl Severe: < 36 mg/dl Cohen , 16 patients (mean Patients with Experienced with Tayco, Disetronic Regular insulin Participants No. CSII Review of glucose diary 24 2003(2) age 14, range 14-18) hypoglycemia MDI, new to CSII pump using and insulin NPH received 2 weeks of patients at each clinic visit. with DM1 for > 2 unawareness were insulin lispro at bedtime training post had 1 extra years; mean A1c excluded randomization visit 2 days 8.5% (CSII patients after start received 3 days of of CSII. Definition of additional training) hypoglycemia: NR DiMeglio, 42 patients (mean No exclusions based on Some experienced Medtronic Insulin lispro and CSII patients No. CSII Patients were to report 26 2004(3) age 3.8, range 1.8- hypoglycemia with MDI, new to MiniMed 508 insulin NPH (2 received 2 training patients episodes of severe 4.7) with DM1 of experience CSII using insulin patients received sessions separated were hypoglycemia mean duration 1.8 lispro insulin Lente, and by 2 weeks and had contacted throughout the study years; mean A1c 1 insulin glargine) “dummy pump.” daily for period; minor 8.8%. Parents wore pump first 10 hypoglycemia was with saline days after assessed for the 2 weeks Definition of infusions for 3 days run in prior to scheduled visits hypoglycemia: during a 2 week run ended. from logbook review and Glucose <60 mg/dl in period. After this meter download period, however, both groups were contacted weekly regardless of means of insulin delivery. Doyle, 32 patients (mean No exclusions based on Few experienced Medtronic Insulin aspart and Participants Yes Patient report of severe 16 2004(4) age 12.5, range 8 to hypoglycemia with MDI (19%), Minimed 508 or insulin glargine received similar events 21) with DM1 of experience otherwise new to Paradigm 511 levels of training (2 mean duration 6.2 MDI or CSII using insulin weeks) after years; mean A1c aspart (with randomization 8.2% multiple basal rates) Definition of hypoglycemia: hypoglycemia resulting in coma or seizure or any other unexpected adverse events Fox, 2005(5) 26 patients (mean No exclusions based on Experienced with Medtronic Insulin lispro or Patients on CSII Yes Download of the 24 age 3.9, range 1-6) hypoglycemia MDI, new to CSII MiniMed 508; aspart; insulin received 2 to 4 glucometer data at each with DM1 of mean experience insulin lispro or NPH weeks of training visit duration 1.5 years, aspart mean A1c 7.5%
Definition of mild/moderate hypoglycemia: glucose < 80/70/60/50 mg/dl Hirsch, 100 patients (mean Patients with a history of Experienced with Multiple pumps Insulin aspart and Participants Yes Logbook review 5 2005(6) age 43, standard severe hypoglycemia or CSII for at least 3 (those patients insulin glargine received 1 week of monthly deviation 11) with hypoglycemia months prior to the were using at training before DM1 of mean unawareness were trial enrollment) using randomization duration 22 years; excluded insulin aspart mean A1c 7.5
Definition of minor hypoglycemia: any asymptomatic blood glucose measurement <50 mg/dl, or as episodes with symptoms consistent with hypoglycemia with confirmation by blood glucose measurement <50 mg/dl that were handled by the subject.
Major hypoglycemia: episodes with severe central nervous system symptoms consistent with hypoglycemia that the patient was unable to treat himself/herself, which had either 1) blood glucose <50 mg/dl or 2) reversal of symptoms after either food intake or glucagon/ intravenous glucose administration Hoogma, 272 patients (mean Patients with a history of Experienced with Disetronic H- Insulin lispro and Participants Yes Glucose diary review 26 2006(7) age 36, range 18-65) severe hypoglycemia or MDI, new to CSII TRON V100 or insulin NPH received 8 weeks of (patient-defined with DM1 of mean hypoglycemia H-TRONplus training post episodes) duration 15 years; unawareness were V100 using randomization mean A1c 7.9 excluded insulin lispro
Definition of hypoglycemia: subject defined (mild: Self treated, and severe: requiring third party help) Lepore, 32 patients (mean No exclusions based on Experienced with Minimed 508 Insulin lispro, Patients received Yes Patients report of severe 50 2003(8) age 40) with DM1 of hypoglycemia MDI (using insulin (n=8), H-Tron insulin glargine equal training in IIT events mean duration 17.5 experience: 4 Patients NPH) Plus Disetronic in both groups yr; mean HbA2c, had history of severe (n=8), insulin (author report) 8.8% Hypoglycemia lispro
Definition of hypoglycemia: NR Opipari- 16 patients (mean No exclusions based on Experienced with Animas pump Insulin lispro and Participants in the Yes Hypoglycemia events 24 Arrigan, age 4.4, range 3.1- hypoglycemia MDI, new to CSII using insulin insulin NPH CSII group reviewed at each 2007(9) 5.3) with >= yr of experience lispro (except for 1 received a 3-h monthly visit by parent DM1; mean HbA1c, patient on lispro training session on report 8.1% and glargine) basal-bolus therapy and had 4 wk of Definition of severe experience with this hypoglycemia: program before Seizure, obtundation receiving pump. or combativeness Participants in the preventing MDI group got administration of oral similar contact glucose in hours but education association with was on family capillary blood management of glucose of less than hyper- and 100 mg/dl hypoglycemia and carbohydrate counting Pozzilli, 23 patients (mean No exclusions based on New to intensive Medtronic Regular insulin Participants No* Logbook review and 24 2003(10) age 18, range 12-35) hypoglycemia insulin therapy MiniMed 507C and insulin NPH received similar meter download with DM1 for < 1 experience using insulin at bedtime. training in intensive month; mean A1c 11 lispro. insulin therapy in both groups (author Definition of report) hypoglycemia: NR Thomas, 21 patients (mean Patients had to have >=1 71% experienced Medtronic 508 Insulin lispro and Similar training in Patients Glucose diary and meter 2007(11) age 43), with DM1 of episode of severe with MDI with insulin lispro insulin glargine both arms randomized download at wk 1, 4, and mean duration 25 yr; hypoglycemia in the last to CSII had every 4 wk mean HbA1c, 8.5% 6 months and evidence ine of hypoglycemia additional Definition of unawareness session hypoglycemia: focused on Glucose < 70 mg/dl the technical aspects of using the pump Weintrob, 23 patients (mean No exclusions based on Experienced with Medtronic Regular insulin Participants No, Glucose diary review at 12 2003-4(12, age 12, range 9-14) hypoglycemia MDI, new to CSII MiniMed 508 and insulin NPH received 2 weeks of patients each clinic visit (every 3 13) with DM1 of mean experience using insulin at bedtime training post randomized to 6 weeks) duration 6 years; lispro. randomization to CSII had mean A1c 8.2 an extra visit Definition of hypoglycemia: Hypoglycemia was defined as symptoms relieved by the ingestion of glucose or food and/or a capillary blood glucose level of < 70 mg/dl
Severe hypoglycemia: any hypoglycemic event requiring assistance from another person or resulting in seizure or coma Wilson 22 patients (mean No exclusions based on Experienced with Medtronic Insulin lispro or Participants Yes Ascertained at each visit 52 2005(14) age 3.6, range 1.7- hypoglycemia MDI, new to CSII MiniMed 508 aspart as prandial received training 6.1) with DM1 of experience using insulin insulin; several before and 2 weeks mean duration 1.4 lispro. insulins used as post randomization years; mean a1c 8% basal insulin.
Definition of hypoglycemia: Glucose < 70.2 mg/dl Hermanides, 83 patients (mean No exclusions based on Experienced with Medtronic multiple daily Patients were Yes Continuous 26 2011(15) age of 38.3, range hypoglycemia MDI, sensor MiniMed Inc injection therapy trained to use the Glucose Sensor 18-65) with DM1 of experience augmented pump. with rapid-acting device within 2 mean duration of 1 insulin analogue weeks after year ; mean HbA1c before meals and randomization 8.56% long-acting analogues or Definition of human insulin hypoglycemia: glucose level < 72 mg/dl
Severe hypoglycemia: glucose of <50 mg/dl resulting in seizure or coma, intravenous glucose or glucagone or any third party assestance
Bergenstal 485 patients (mean Exclusions included use Experienced with MiniMed The injection- During the 5 weeks No, Sensor glucose values 52 2010(16) age of 32.2, SD 17.5 of insulin pump therapy MDI, new to CSII Paradigm REAL- therapy group after patients were collected for 1- in SAP, 31.5+/-16.5 within the previous 3 Time System, used both insulin randomization, randomized week periods at baseline, in MDI); mean years, a history of at Medtronic) glargine (Lantus, patients in the to CSII had 6 months, and 1 year in HbA1c 8.3+/-0.5 least two severe Sanofi-Aventis) pump-therapy extra visits the two study groups. SAP, 8.3+/-0.5 MDI hypoglycemic events in and insulin aspart group completed % the year before under the online insulin-pump enrollment, the use of a guidance of the training and Definition of pharmacologic treating clinician. attended additional Hypoglycemia: noninsulin treatment for visits for insulin- glucose <70 mg/dl diabetes during the pump and sensor previous 3 months, training. Peyrot, 28 patients (mean No exclusions based on Experienced with Paradigm® 722 NR Participants in both NR assessed at baseline and 16 2009(17) age of 47.2, range hypoglycemia MDI, new to CSII System, arms received 2–4 h end of study 25-70) with DM1 of experience Medtronic of education mean duration of 25 MiniMed, regarding diet, years ; mean HbA1c Northridge, CA exercise, BG and 8.6% acute crisis management, BG Definition of and ketone testing, hypoglycemia: NR and use of the DMS. Participants in the study arm also received 4–5 h of education regarding use of the RT-CGM/CSII system Little, 96 patients (mean No exclusions based on Experienced with Paradigm Veo aspart/glargine Prior to Yes Participants recorded SH 24 2014(18) age of 48.6 +/-12.2 hypoglycemia MDI, new to CSII insulin pump; randomization, all (severe hypoglycemia) years) with DM1 of experience Medtronic participants episodes prospectively mean duration of 29 attended a single 1- and were recalled every years ; mean HbA1c to 2-h standardized 4 weeks up to 24 weeks 8.2% education session. Also included was Definition of advice on self- hypoglycemia: adjustment of glucose < 54 mg/dl insulin doses according to carbohydrate intake, SMBG, and planned activity and recommendation for oral carbohydrate administration for all glucose levels,4.0mmol/L. Bolli, 58 patients (age Patients who had more Experienced with NR Insulin glargine NR Yes Participants were asked 24 2009(19) range 18-70, mean than 2 severe MDI, new to CSII plus meal time to perform self- age of 37.6 +/-12.3 in hypoglycemic events in insulin lispro. monitored plasma CSII, 42.4 +/-9.9 in previous 6 months were glucose measurements MDI) with DM1 excluded. four times daily (bedtime duration of more than and preprandial) using a 1 year; mean HbA1c plasma- calibrated 7.7% in CSII, 7.8% memory glucose meter. in MDI. Hypoglycemia was recorded in diaries and Definition of extracted at study visits. hypoglycemia: symptoms consistent with hypoglycemia not requiring the assistance of another person and confirmed by plasma glucose <72 mg/dl
Severe hypoglycemia: similar symptoms but requiring management assistance and either plasma glucose <36 mg/dl
Thrailkill 24 patients (age No exclusions based on New to intensive IR 1250 Lantus or NPH NR Yes Continuous 52 2011(20) range 8-18, mean age hypoglycemia insulin therapy Glucose Sensor of 12.1 in both experience groups)
Definition of hypoglycemia: Glucose < 70 mg/dl Skogsberg 72 patients (mean No exclusions based on New to intensive H-Tron NPH and aspart During The Data downloaded from 104 2008(21) age 11.8 in CSII hypoglycemia insulin therapy hospitalization, all patients in the pump. For the MDI group, 12.3 in MDI experience patients and their the CSII group, Ha1c was group) parents were group checked during visits educated and received an Definition of severe trained by the additional hypoglycemia: pediatric diabetes half a day episodes requiring team according to of assistance for the National education recovery from guidelines in another person operating the insulin pump. Nuboer 39 patients (mean No exclusions based on New to intensive NR NPH and insulin NR NR NR 56 2008(22) age 10, range 4-16)) hypoglycemia insulin therapy glargine experience Definition of hypoglycemia: NR Nabhan, 42 patients (mean No exclusions based on New to intensive NR NR Families had a half- Yes All families were asked 52 2009(23) age: 3.7±0.8 years, hypoglycemia insulin therapy day education to monitor blood glucose HbA1c: 8.9±0.6) experience session during levels prior to each meal which insulin types, and at bedtime, using the Definition of adjustment and Accuchek Complete hypoglycemia: NR carbohydrate meter (Roche counting were Diagnostics, reviewed. Indianapolis, Ind) Rabbone 40 patients (mean No exclusions based on New to intensive NR NPH and rapid- NR NR NR 52 2008(24) age 3.2 in CSII, 3.9 hypoglycemia insulin therapy acting analog in MDI group) experience
Definition of severe hypoglycemia: the presence of seizures or clinical condition resulting in unconsciousness Roselund 57 patients (mean Patients with severe Experienced with Paradigm Veo and Rapid acting All patients in both NR Patients were monitored 52 2015(25) age 51±10m DM recurrent hypoglycemia MDI, some are new Enlite sensor insulin randomization using CGM duration 33±12 were excluded to CSII (Medtronic, Inc.) groups trained in years) diabetes self-care by an educated Definition of diabetes nurse and hypoglycemia: were examined by glucose <70.2 mg/dl the same doctor and nurse during the study Perkins 329 adult patients Patients with history of (MiniMed Insulin aspart, and All subjects Yes Patients were monitored 52 2015(26) (271 in the US, mean more than 1 severe Paradigm, glargine received training in using CGM and were age 41.3±21.3 and 58 hypoglycemic event in Medtronic intensive diabetes scheduled for visits each in Canada, mean age the year before management 3 months 40.7±11.8) enrolment were excluded Definition of hypoglycemia: NR
References
1. Bruttomesso D, Crazzolara D, Maran A, Costa S, Dal Pos M, Girelli A, et al. In Type 1 diabetic patients with good glycaemic control, blood glucose variability is lower during continuous subcutaneous insulin infusion than during multiple daily injections with insulin glargine. Diabetic medicine : a journal of the British Diabetic Association. 2008;25(3):326-32. doi: 10.1111/j.1464-5491.2007.02365.x. 2. Cohen D, Weintrob N, Benzaquen H, Galatzer A, Fayman G, Phillip M. Continuous subcutaneous insulin infusion versus multiple daily injections in adolescents with type I diabetes mellitus: a randomized open crossover trial. J Pediatr Endocrinol Metab. 2003;16(7):1047-50. 3. DiMeglio LA, Pottorff TM, Boyd SR, France L, Fineberg N, Eugster EA. A randomized, controlled study of insulin pump therapy in diabetic preschoolers. The Journal of pediatrics. 2004;145(3):380-4. doi: 10.1016/j.jpeds.2004.06.022. 4. Doyle EA, Weinzimer SA, Steffen AT, Ahern JA, Vincent M, Tamborlane WV. A randomized, prospective trial comparing the efficacy of continuous subcutaneous insulin infusion with multiple daily injections using insulin glargine. Diabetes Care. 2004;27(7):1554-8. 5. Fox LA, Buckloh LM, Smith SD, Wysocki T, Mauras N. A randomized controlled trial of insulin pump therapy in young children with type 1 diabetes. Diabetes Care. 2005;28(6):1277-81. 6. Hirsch IB, Bode BW, Garg S, Lane WS, Sussman A, Hu P, et al. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care. 2005;28(3):533-8. 7. Hoogma RP, Hammond PJ, Gomis R, Kerr D, Bruttomesso D, Bouter KP, et al. Comparison of the effects of continuous subcutaneous insulin infusion (CSII) and NPH-based multiple daily insulin injections (MDI) on glycaemic control and quality of life: results of the 5-nations trial. Diabetic medicine : a journal of the British Diabetic Association. 2006;23(2):141-7. doi: 10.1111/j.1464-5491.2005.01738.x. 8. Lepore G, Dodesini AR, Nosari I, Trevisan R. Both continuous subcutaneous insulin infusion and a multiple daily insulin injection regimen with glargine as basal insulin are equally better than traditional multiple daily insulin injection treatment. Diabetes Care. 2003;26(4):1321-2. 9. Opipari-Arrigan L, Fredericks EM, Burkhart N, Dale L, Hodge M, Foster C. Continuous subcutaneous insulin infusion benefits quality of life in preschool-age children with type 1 diabetes mellitus. Pediatr Diabetes. 2007;8(6):377-83. doi: 10.1111/j.1399-5448.2007.00283.x. 10. Pozzilli P, Crino A, Schiaffini R, Manfrini S, Fioriti E, Coppolino G, et al. A 2-year pilot trial of continuous subcutaneous insulin infusion versus intensive insulin therapy in patients with newly diagnosed type 1 diabetes (IMDIAB 8). Diabetes Technol Ther. 2003;5(6):965-74. doi: 10.1089/152091503322641006. 11. Thomas RM, Aldibbiat A, Griffin W, Cox MA, Leech NJ, Shaw JA. A randomized pilot study in Type 1 diabetes complicated by severe hypoglycaemia, comparing rigorous hypoglycaemia avoidance with insulin analogue therapy, CSII or education alone. Diabetic medicine : a journal of the British Diabetic Association. 2007;24(7):778-83. doi: 10.1111/j.1464-5491.2007.02196.x. 12. Weintrob N, Benzaquen H, Galatzer A, Shalitin S, Lazar L, Fayman G, et al. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens in children with type 1 diabetes: a randomized open crossover trial. Pediatrics. 2003;112(3 Pt 1):559-64. 13. Weintrob N, Schechter A, Benzaquen H, Shalitin S, Lilos P, Galatzer A, et al. Glycemic patterns detected by continuous subcutaneous glucose sensing in children and adolescents with type 1 diabetes mellitus treated by multiple daily injections vs continuous subcutaneous insulin infusion. Arch Pediatr Adolesc Med. 2004;158(7):677-84. doi: 10.1001/archpedi.158.7.677. 14. Wilson DM, Buckingham BA, Kunselman EL, Sullivan MM, Paguntalan HU, Gitelman SE. A two-center randomized controlled feasibility trial of insulin pump therapy in young children with diabetes. Diabetes Care. 2005;28(1):15-9. 15. Hermanides J, Norgaard K, Bruttomesso D, Mathieu C, Frid A, Dayan CM, et al. Sensor-augmented pump therapy lowers HbA(1c) in suboptimally controlled Type 1 diabetes; a randomized controlled trial. Diabet Med. 2011;28(10):1158-67. doi: http://dx.doi.org/10.1111/j.1464- 5491.2011.03256.x. 16. Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes.[Erratum appears in N Engl J Med. 2010 Sep 9;363(11):1092]. N Engl J Med. 2010;363(4):311-20. doi: http://dx.doi.org/10.1056/NEJMoa1002853. 17. Peyrot M, Rubin RR. Patient-reported outcomes for an integrated real-time continuous glucose monitoring/insulin pump system. Diabetes Technol Ther. 2009;11(1):57-62. doi: http://dx.doi.org/10.1089/dia.2008.0002. 18. Little SA, Leelarathna L, Walkinshaw E, Tan HK, Chapple O, Lubina-Solomon A, et al. Recovery of hypoglycemia awareness in long- standing type 1 diabetes: A multicenter 2 x 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care. 2014;37(8):2114-22. 19. Bolli GB, Kerr D, Thomas R, Torlone E, Sola-Gazagnes A, Vitacolonna E, et al. Comparison of a multiple daily insulin injection regimen (basal once-daily glargine plus mealtime lispro) and continuous subcutaneous insulin infusion (lispro) in type 1 diabetes: a randomized open parallel multicenter study. Diabetes Care. 2009;32(7):1170-6. 20. Thrailkill KM, Moreau CS, Swearingen C, Rettiganti M, Edwards K, Morales AE, et al. Insulin pump therapy started at the time of diagnosis: effects on glycemic control and pancreatic beta-cell function in type 1 diabetes. Diabetes Technol Ther. 2011;13(10):1023-30. doi: http://dx.doi.org/10.1089/dia.2011.0085. 21. Skogsberg L, Fors H, Hanas R, Chaplin JE, Lindman E, Skogsberg J. Improved treatment satisfaction but no difference in metabolic control when using continuous subcutaneous insulin infusion vs. multiple daily injections in children at onset of type 1 diabetes mellitus. Pediatr Diabetes. 2008;9(5):472-9. doi: http://dx.doi.org/10.1111/j.1399-5448.2008.00390.x. 22. Nuboer R, Borsboom GJ, Zoethout JA, Koot HM, Bruining J. Effects of insulin pump vs. injection treatment on quality of life and impact of disease in children with type 1 diabetes mellitus in a randomized, prospective comparison. Pediatr Diabetes. 2008;9(4 Pt 1):291-6. doi: http://dx.doi.org/10.1111/j.1399-5448.2008.00396.x. 23. Nabhan ZM, Kreher NC, Greene DM, Eugster EA, Kronenberger W, DiMeglio LA. A randomized prospective study of insulin pump vs. insulin injection therapy in very young children with type 1 diabetes: 12-month glycemic, BMI, and neurocognitive outcomes. Pediatr Diabetes. 2009;10(3):202-8. doi: http://dx.doi.org/10.1111/j.1399-5448.2008.00494.x. 24. Rabbone I, Bobbio A, Di Gianni V, Sacchetti C, Cerutti F. Intensive insulin therapy in preschool-aged diabetic children: from multiple daily injections to continuous subcutaneous insulin infusion through indwelling catheters. J Endocrinol Invest. 2008;31(3):193-5. 25. Rosenlund S, Hansen TW, Rossing P, Andersen S. Effect of Sensor-Augmented Pump Treatment Versus Multiple Daily Injections on Albuminuria: A 1-Year Randomized Study. The Journal of clinical endocrinology and metabolism. 2015;100(11):4181-8. doi: 10.1210/jc.2015- 2839. 26. Perkins BA, Halpern EM, Orszag A, Weisman A, Houlden RL, Bergenstal RM, et al. Sensor-augmented pump and multiple daily injection therapy in the United States and Canada: post-hoc analysis of a randomized controlled trial. Canadian Journal of Diabetes. 2015;39(1):50-4. doi: 10.1016/j.jcjd.2014.03.003.