Chemistry 332 Aromatic Molecules: Structure and Reactions
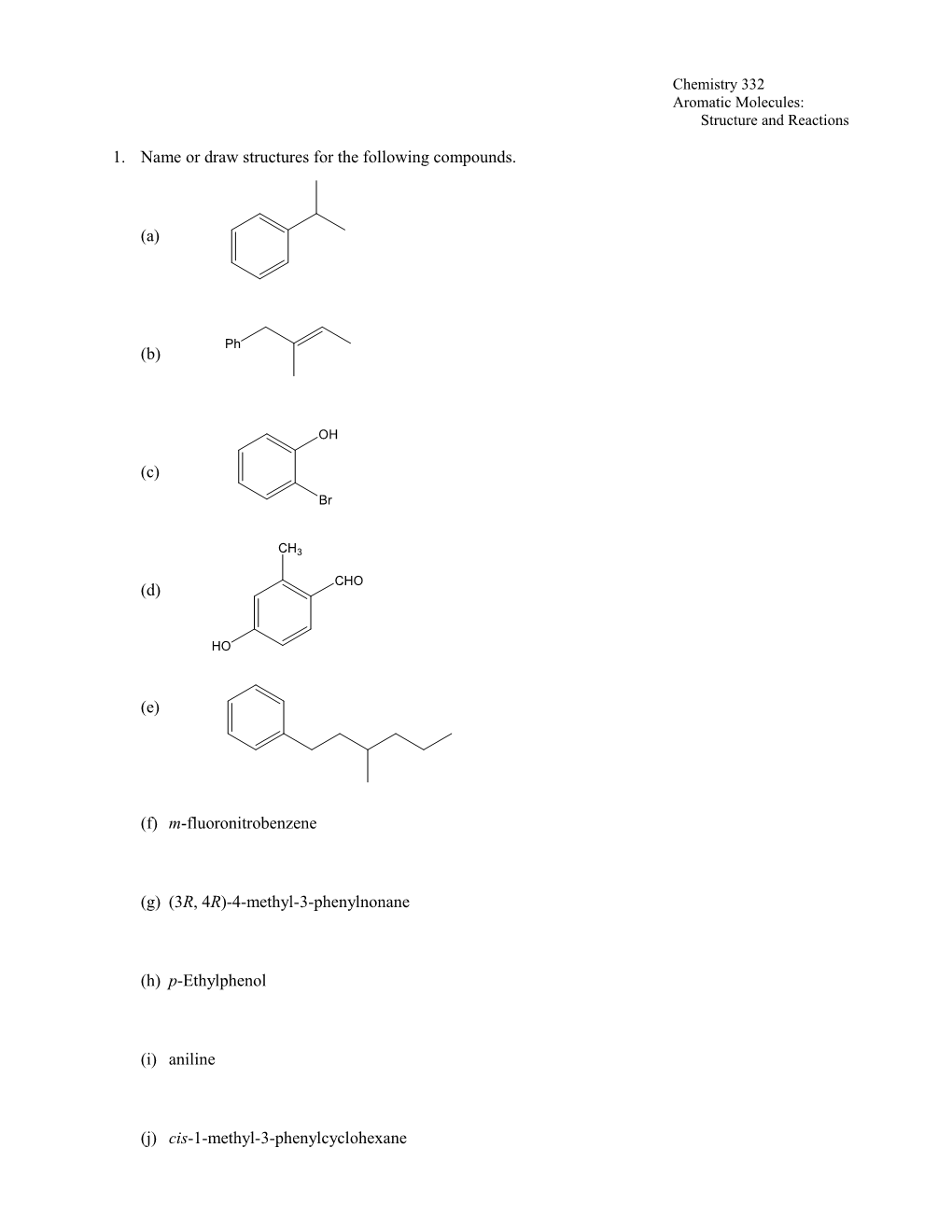
1. Name or draw structures for the following compounds.
(a)
Ph (b)
OH
(c)
Br
CH3
CHO (d)
HO
(e)
(f) m-fluoronitrobenzene
(g) (3R, 4R)-4-methyl-3-phenylnonane
(h) p-Ethylphenol
(i) aniline
(j) cis-1-methyl-3-phenylcyclohexane Chemistry 332 Aromatic Molecules: Structure and Reactions
2. Classify each of the following compounds as aromatic, antiaromatic, or nonaromatic.
(a)
(b)
(c)
(d)
O (e) N
N H
OH (f)
O
(g) [18]annulene Chemistry 332 Aromatic Molecules: Structure and Reactions
3. Predict the major organic product(s) of each of the following reactions. If no reaction will occur, write “N.R.”
NO2 (a) CH3Cl AlCl3
Cl (b) 2 FeCl3
(c) HNO3
H2SO4
4. Provide reagents by each arrow below to complete the following reaction scheme.
Br
O OH Chemistry 332 Aromatic Molecules: Structure and Reactions
5. (i) Draw the major product of the following reaction.
HNO3
H2SO4
(ii) Draw the major product formed from the reaction of the compound you drew in part (i) with another electrophile (E+).
(iii) Draw the intermediate of the reaction described in part (ii). Include all valid, contributing resonance structures of this intermediate in your answer.
(iv) Explain why the isomer you drew in part (ii) is the major product of this reaction (rather than any other isomers). Chemistry 332 Aromatic Molecules: Structure and Reactions
6. Circle the letter that corresponds to the correct answer for each question. There is only one correct answer for each question.
(i) Which of the statements is TRUE regarding the nitration reaction of isopropylbenzene? A. A 50:50 mixture of ortho and para products will be formed. B. A greater amount of the para product will be formed. C. A greater amount of the ortho product will be formed D. Only the meta product will be formed.
(ii) Which of the following compounds would undergo the fastest Friedel-Crafts reaction? A. Nitrobenzene B. Aniline C. Benzaldehyde D. Benzene
(iii) Which of the following sets of substituents are ALL ortho/para directing groups in electrophilic aromatic substitution?
A. Cl, CN, NO2 B. CH3, OCH3, C(O)CH3 C. Cl, NH2, CH3 D. CN, NO2, C(O)CH3
7. Aniline reacts with nitrous acid, HNO2, to yield a stable diazonium salt.
This diazonium salt undergoes electrophilic aromatic substitution on activated aromatic rings to yield brightly colored azo compounds that are widely used as dyes. The intermediate structures for the mechanism of this reaction are given below. Show all electron flow with arrows for this mechanism on the structures provided.