Unit 12 WATER and SOLUTIONS!!! Let’s talk about solutions a bit more in depth 1) A solution is made of two parts, name them and define each (pg. 482)
2) Describe the solvation process (pg. 483)
3) What kind of things dissolve in water (sometimes called the universal solvent)?
4) What does “like dissolves like” mean?
5) What are some things that determine how fast a solute will dissolve in a solvent (pg. 501,502)
6) Solubility (pg. 502-507) a. What are the three levels of solubility and describe each.
b. What is the most common factor that changes solubility? i. How does this factor usually effect how solids dissolve in water?
ii. How does this factor usually effect how gases dissolve in water?
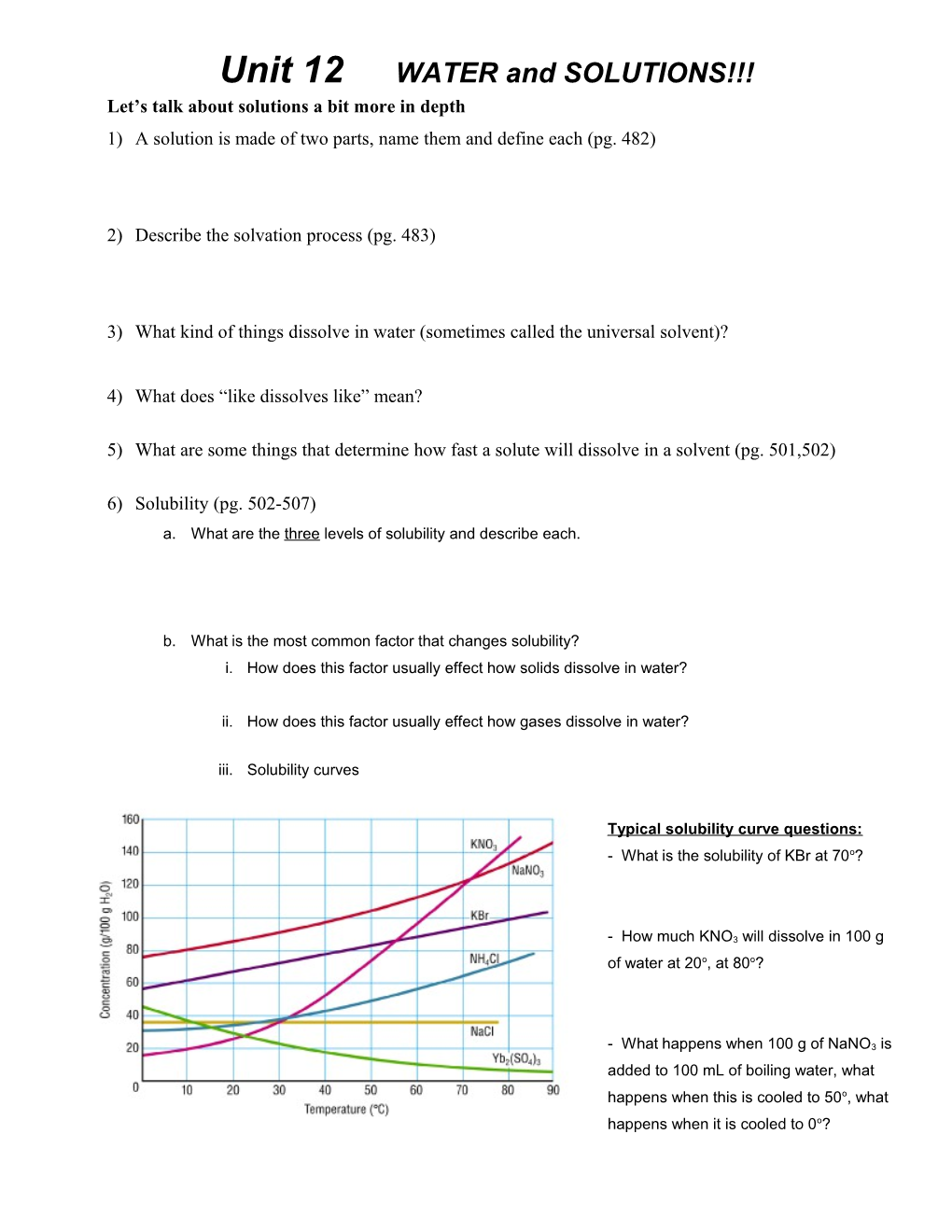
iii. Solubility curves
Typical solubility curve questions: - What is the solubility of KBr at 70o?
- How much KNO3 will dissolve in 100 g of water at 20o, at 80o?
- What happens when 100 g of NaNO3 is added to 100 mL of boiling water, what happens when this is cooled to 50o, what happens when it is cooled to 0o? Concentration Units: 1) Molarity (M) = moles of solute/ L of solution (pg.509-513) a. From a solid: What is the M of a solution of 10 g of NaCl in enough water to make 250 mL of solution?
b. Dilution: (M1V1 = M2V2) I take 25.0 mL of 12M HCl and dilute it to 500 mL with water, what is the solutions new M?
How do I make 250.0 mL of 1.0M nitric acid from a stock solution (15M)?
2) Percentages: (pg. 513-514) a. Percent by mass = mass solute/ total mass
b. Percent by volume = volume of solute/ total volume of solution
c. Percent mass/volume = mass of solute (g) / total volume of solution (mL)
Summary Problem: I dissolved 25.0 mL of C2H5OH (D = 0.82 g/mL) in 75.0 mL of water. The resulting solution had a volume of 97.0 mL. Give the concentration of this solution in all 4 units we’ve learned.
solute solvent solution mass volume moles