Comments/suggestions for Kinetics laboratory exercise write-up:
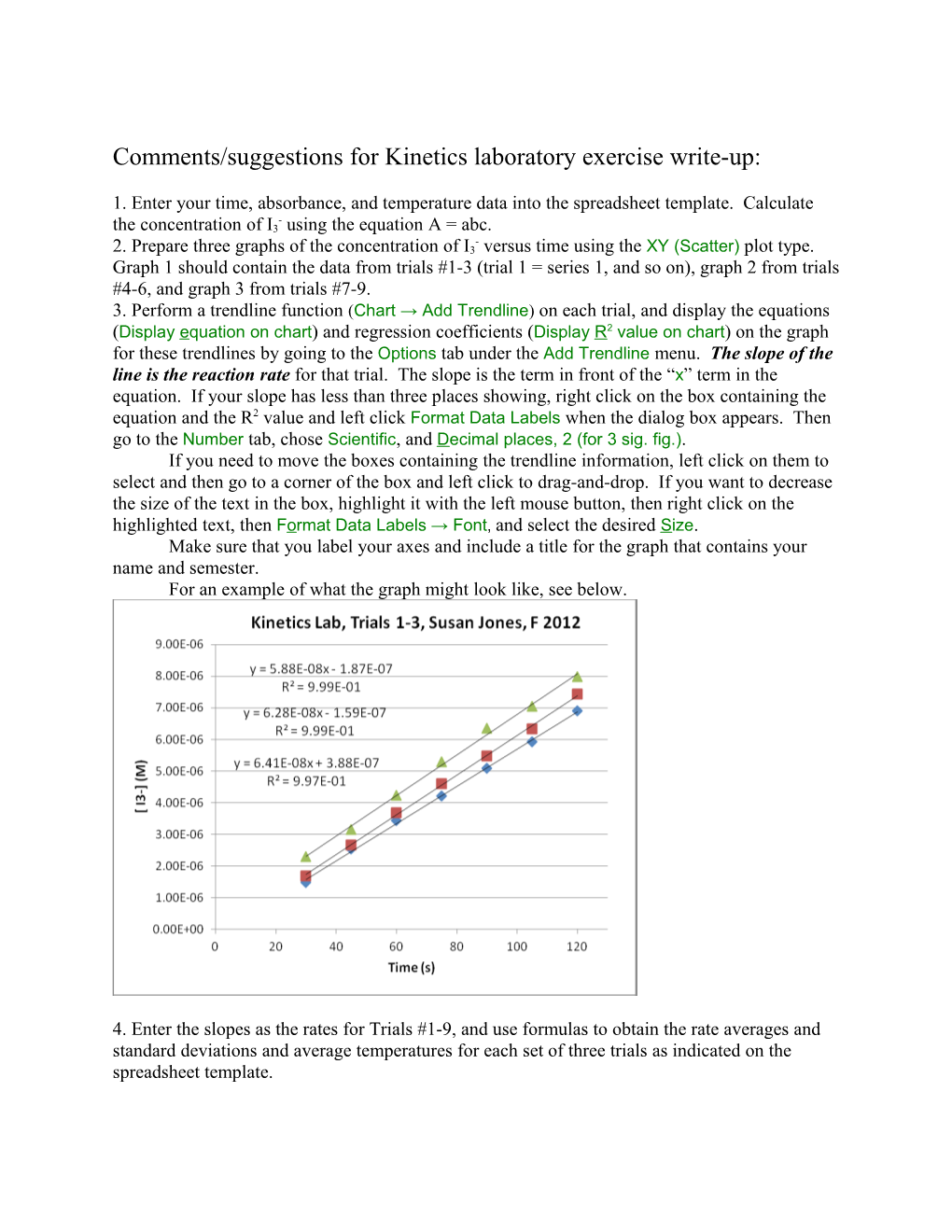
1. Enter your time, absorbance, and temperature data into the spreadsheet template. Calculate - the concentration of I3 using the equation A = abc. - 2. Prepare three graphs of the concentration of I3 versus time using the XY (Scatter) plot type. Graph 1 should contain the data from trials #1-3 (trial 1 = series 1, and so on), graph 2 from trials #4-6, and graph 3 from trials #7-9. 3. Perform a trendline function (Chart → Add Trendline) on each trial, and display the equations (Display equation on chart) and regression coefficients (Display R2 value on chart) on the graph for these trendlines by going to the Options tab under the Add Trendline menu. The slope of the line is the reaction rate for that trial. The slope is the term in front of the “x” term in the equation. If your slope has less than three places showing, right click on the box containing the equation and the R2 value and left click Format Data Labels when the dialog box appears. Then go to the Number tab, chose Scientific, and Decimal places, 2 (for 3 sig. fig.). If you need to move the boxes containing the trendline information, left click on them to select and then go to a corner of the box and left click to drag-and-drop. If you want to decrease the size of the text in the box, highlight it with the left mouse button, then right click on the highlighted text, then Format Data Labels → Font, and select the desired Size. Make sure that you label your axes and include a title for the graph that contains your name and semester. For an example of what the graph might look like, see below.
4. Enter the slopes as the rates for Trials #1-9, and use formulas to obtain the rate averages and standard deviations and average temperatures for each set of three trials as indicated on the spreadsheet template.