Chapter 2. Intermolecular Forces and Thermodynamic Models
Problem 1. Basic calculations i. For a zwitterionic species e.g. amino acids for which the charge q is equal to the electronic (elementary) charge and the charge separation l = 0.1nm , calculate the dipole moment in both Debye and Cm. ii. A good approximation for the ionization potential and the (volume-based) electronic polarizability are for many molecules around 2x10-18 J and 1.5x10-30 m3. Show that for two such molecules in contact ( r = 0.3nm ) at room temperature, the dispersion potential is approximately equal to kT . iii. Based on the group values for dipole moments and electronic polarizabilities given in Tables 2.10 and 2.11: - calculate the orientational polarizability for a molecule having a dipole moment equal to 1 D - calculate the electronic polarizability of methanol and compare the value to that reported in Table 2.10
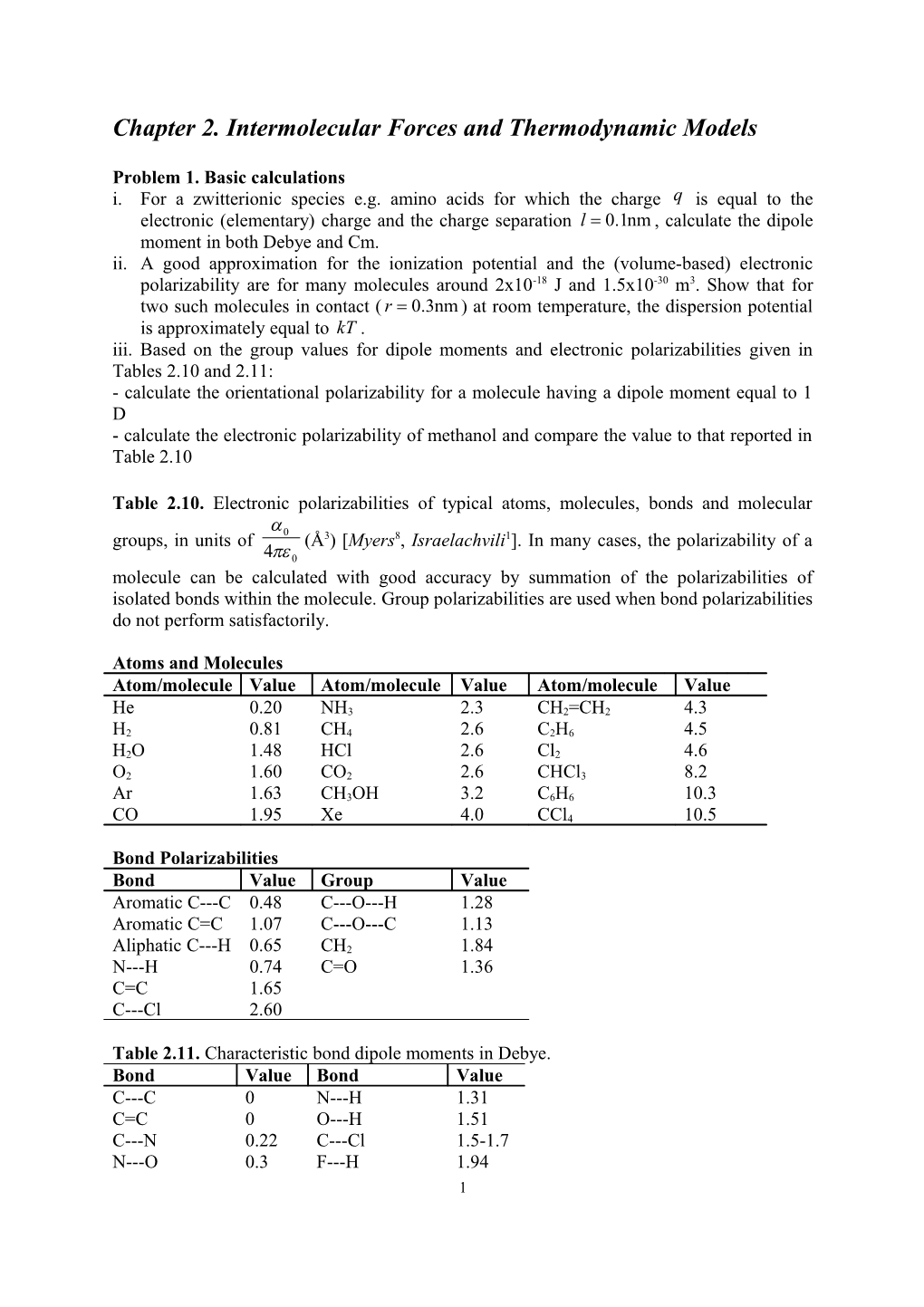
Table 2.10. Electronic polarizabilities of typical atoms, molecules, bonds and molecular a groups, in units of 0 (Å3) [Myers8, Israelachvili1]. In many cases, the polarizability of a 4pe 0 molecule can be calculated with good accuracy by summation of the polarizabilities of isolated bonds within the molecule. Group polarizabilities are used when bond polarizabilities do not perform satisfactorily.
Atoms and Molecules Atom/molecule Value Atom/molecule Value Atom/molecule Value
He 0.20 NH3 2.3 CH2=CH2 4.3 H2 0.81 CH4 2.6 C2H6 4.5 H2O 1.48 HCl 2.6 Cl2 4.6 O2 1.60 CO2 2.6 CHCl3 8.2 Ar 1.63 CH3OH 3.2 C6H6 10.3 CO 1.95 Xe 4.0 CCl4 10.5
Bond Polarizabilities Bond Value Group Value Aromatic C---C 0.48 C---O---H 1.28 Aromatic C=C 1.07 C---O---C 1.13 Aliphatic C---H 0.65 CH2 1.84 N---H 0.74 C=O 1.36 C=C 1.65 C---Cl 2.60
Table 2.11. Characteristic bond dipole moments in Debye. Bond Value Bond Value C---C 0 N---H 1.31 C=C 0 O---H 1.51 C---N 0.22 C---Cl 1.5-1.7 N---O 0.3 F---H 1.94 1 C---H 0.4 N=O 2.0 C---O 0.74 C=O 2.3-2.7
Problem 2. London forces from different expressions Consider two identical molecules interacting in free space. Compare the London contribution from Lifshitz theory (equation 2.7) to the expression for the dispersion forces from Table 2.1. What do you observe? Are the two expressions identical?
Problem 3. Relative importance of polar and dispersion forces for water/alkanes The refractive index of methane is 1.30, while that of water is 1.33. The dielectric constant of water is about 80 and that of alkanes about 2. i. Show that for two methane molecules in water, the major contribution of the van der Waals forces comes from the polar (zero-frequency) term and that equation 2.7 reduces approximately to: kTR 3 1 0 0 0 r 6 2.23 ii. Experimental measurements on hydrophobic interactions e.g. alkane (here methane) attractions in water have been reported to be about 10 kJ/mol at 25 oC. How does this value compare to the one calculated from the previous equation? Comment on the results.
Problem 4. Coulombic and Polar van der Waals forces
4.1 Consider two ions Cl- and Na+ at contact ( r = 0.276nm ) and also at a distance equal to r = 56nm . i. What is the inter-ion potential energy at both distances and both in vacuum and in water o (at 25 C when the relative permittivity of water is equal to r 78.41)? ii. Calculate the potential energies of question (i) in both J (per molecule) and kJ/mol and as fractions of kT (at room temperature) where k is the Boltzmann constant. How do these energies compare to the kinetic energy (=3/2 kT ) and to the hydrogen-bonding energy (about 20 kJ/mol)? Comment on the results.
4.2 Two small identical polar molecules e.g. HCl with dipole moment equal to 1 D at 25 oC are in a separation of about 0.5 nm. i. What is the value of potential energy in kJ/mol and how does it compare to the kinetic energy at the same temperature? ii. What is the dipole moment of unit charges (e=1.60218x10-19 C) at a distance of 0.1 nm? Is this dipole moment value higher or lower than that of polar molecules like water or acetone? Comment on the results.
Problem 5. The origin of the geometric-mean rule in the cross interactions for cubic equations of state Starting from the London expression for the intermolecular potential show that the attractive
2 cross intermolecular potential between two unlike non-polar molecules 1 and 2 ( 12 ) is given by the equation: 2 I I 1 2 12 1 2 I I 1 2 2.24 where i indicates the potential energy between two identical molecules type i and Ii is the ionization potential of molecule i.
Problem 6. From the cross intermolecular potential to combining rules The Mie potential function is given by the equation: n m n m- n 轾 m骣 m 骣s12 骣 s 12 G12 =琪 e 12 犏琪 - 琪 m- n桫 n臌犏桫 r 桫 r 2.25 where Ii is the ionization potential of component i, 12 is the molecular cross-energy parameter, 12 is the molecular cross diameter and r is the distance of the molecules. Assume that, as shown by Coutinho et al., Ind. Eng. Chem. Res., 2000, 39(8), 2076, the ionization potential can be, for several systems, approximated as follows: 1 1 2 I I b b I 1 2 1 2 3 I1 I2 b12 2.26
Consider also that the ‘microscopic’ ( , ) and ‘macroscopic’ properties - parameter (
Tc, V c , a , b ) can be related via:
a e 档 T b c 3 s 档b Vc 2.27
Tc, V c , a , b are respectively the critical temperature, critical volume and the energy and co- volume parameters of cubic equations of state. Show, using equations 2.25 - 2.27 and the result from Problem 5, that the following general combining rule can be derived for the energy parameter of cubic equations of state: n -2 骣 b b 3 a= a a 琪 1 2 12 1 2 琪 桫 b12 2.28 For which value of n is the classical geometric-mean rule for the cross energy parameter obtained? Comment on the result.
Problem 7. van der Waals forces between like and unlike molecules
3 Calculate the C-parameters of the van der Waals forces and the percentage (%) contribution of dispersion, induction and polar contributions at two temperatures (273 and 293 K) for: i. two methane molecules ii. two Ne-molecules iii. two water molecules iv. Ne-methane v. Methane-water
Compare the two unlike molecules interaction ( C12 ) parameters to those obtained using the interactions between like molecules and the geometric mean rule (C12 C1C2 ). What do you observe? Discuss briefly the results.
Data needed: Reduced electronic polarizabilities: methane (2.6x10-30), Ne (0.39x10-30), water (1.48x10-30) – all values in m3. Ionization potential: methane (12.6), Ne (21.6), water (12.6) – all values in eV (=1.602x10-19 J). Only water has a dipole moment (=1.85 D).
Problem 8. Second virial coefficients from intermolecular potentials Starting from the relationship between the second virial coefficient and the intermolecular potential, derive the following expressions for the second virial coefficient for the hard- sphere and square-well fluids:
2 B N 3 3 A 2.29
2 3 3 kT B N A 1 1e 1 3 2.30 Which of the two expressions is closer to that of a real fluid?
Problem 9. Multiple choice questions on intermolecular forces Fill in Table 2.11. Only one answer is correct for each question.
Table 2.11. Multiple choice questions. 1. Intermolecular forces are not present… a. In ideal solutions b. In ideal gases c. In the liquid solution polyethylene/hexane d. When we have mixtures with alkanes
2. In which of the following molecules are hydrogen bonds likely to exist? a. n-pentane b. Carbon dioxide c. Ethanol d. Polystyrene
4 3. All three types of ”van der Waals forces”... a. Depend on the dipole moment b. Depend on temperature c. Have the same dependency on intermolecular distance d. Are not important in thermodynamic calculations
4. In terms of their dependency on the intermolecular distance… a. Dispersion forces are the weakest of all b. Ion-ion forces are stronger than dipole-dipole ones c. Quadrupole-quadrupole forces are stronger than dipole-dipole ones d. van der Waals forces are in general stronger than ionic forces
5. Figure 2.10 shows a phase diagram for CO2 - ethane at 223.1 K. The non satisfactory description with SRK may be due to… a. The high polar forces at low temperatures b. The high quadrupolar forces especially at low temperatures c. The fact we have a mixture of two gases d. The pressure being higher than the atmospheric pressure
6. “Intramolecular association” is a special form of hydrogen bonding (HB) indicating… a. … HB between two different molecules of same type b. … HB between two different molecules of different types c. … HB between two atoms inside the same molecule d. … HB between water and another molecule
7. Ethanol and dimethyl ether are isomers but ethanol’s boiling point is 79 oC, while ether’s boiling point is only -25 oC. This large difference is due to… a. The difference in polarity between the two compounds b. The hydrogen bonding character of ethanol c. The difference in the quadrupole moments of the two compounds d. The difference in molecular weight between the two compounds
8. Hydrogen bonding is not at all related to only one of the following phenomena. Which one? a. The DNA double-helix b. The floating of ice on water c. The negative deviations from Raoult’s law in a polyethylene-hexane mixture d. The formation of dimers of organic acids in the vapour phase
9. Which of the following mixtures is not likely to exhibit hydrogen bonding interactions? a. Methanol-water b. Propanol-hexane c. Polyethylene-octane d. Acetic acid – water
10. In which of the following mixtures are expected “cross-association” (solvation) phenomena due to hydrogen bonding interactions? a. Methanol-hexane b. Water-octane 5 c. Chloroform-acetone d. Acetone-octane
11. The intermolecular potential of an ideal gas is… a. Given by the Lennard-Jones equation b. Zero c. Approximated by the square-well equation d. Given by a complex function which is not known
12. At high temperatures … a. Dipole forces become more pronounced b. Hydrogen-bonding interactions become weaker c. Dispersion interactions increase d. There are no intermolecular forces
13. Dispersion forces … a. Depend on the dipole moments of the compounds b. Are always smaller than “dipole-dipole” forces c. Are often very significant d. Depend on temperature
14. Hydrogen bonds are often called “quasi-chemical” (forces) because they…. a. Are equally strong as the “chemical” (covalent) bonds b. Have a strength close to that of chemical bonds c. Are being taught in chemistry courses d. Are bonds between chemicals
15. Which of the following equations do not correspond to a well-known intermolecular potential function? a. Square-well b. Hard-sphere c. Peng-Robinson d. Lennard-Jones
16. Cubic equations of state like SRK together with the classical (van der Waals one fluid) mixing rules do not account explicitly for polar, ionic and hydrogen-bonding effects. They can thus be expected to represent best the phase equilibria of … a. Aqueous hydrocarbon solutions b. Mixtures of hydrocarbons c. Mixtures with electrolytes d. Water-heavy alcohols mixtures (liquid-liquid equilibria)
17. The SAFT equation of state (statistical associating fluid theory) is a complex equation of state derived from statistical thermodynamics and accounts explicitly for a number of effects: dispersion forces, macromolecular character of polymers, hydrogen bonding. However, SAFT cannot satisfactorily describe phase equilibria for acetone-hexane. This is likely to be due to… a. The fact that SAFT is a complex model and fails for simple near-ideal solutions b. The presence of the azeotrope 6 c. The lack of a polar term in the SAFT equation of state d. The rather low temperature of the mixture
18. The virial coefficients of real gases are more likely to be well-represented by… a. The hard-sphere potential function b. The square-well potential function c. The Lennard-Jones potential function d. The ideal gas equation of state
19. The Carnahan-Starling equation is … a. An equation of state better than SRK or Peng-Robinson b. A cubic equation of state c. A model based on a realistic potential for real fluids d. An equation representing the hard-sphere fluid
20. The highest values of virial coefficients are expected for… a. Alcohols b. Organic acids c. Hydrocarbons d. Ideal gases
10
9
8 r a b , P 7
6
5 0 0,2 0,4 0,6 0,8 1 mole fr. ethane
Figure 2.10. Vapor-liquid equilibrium diagram for CO2 - ethane at 223.1 K. The points are the experimental data and the lines are the calculations with the SRK equation of state using a value for the k12 parameter equal to 0.1304.
7