A Radical-Mediated Pathway for the Formation of [M+H]+ in Dielectric Barrier Discharge Ionization
Jan-Christoph Wolf*‡, Luzia Gyr‡, Mario F. Mirabelli, Martin Schaer†, Peter
Siegenthaler† and Renato Zenobi*
Department of Chemistry and Applied Bioscience, ETH Zurich, CH-8093 Zurich, Switzerland. † Federal Office for Civil Protection FOCP, SPIEZ LABORATORY, Analytical Chemistry Branch, CH-3700 Spiez, Switzerland. ‡ These authors contributed equally
AUTHOR INFORMATION Corresponding Authors Prof. Dr. Renato Zenobi, D-CHAB, ETH Zurich, CH-8093 Zurich, Switzerland. Email: [email protected] Dr. Jan-Christoph Wolf, D-CHAB, ETH Zurich, CH-8093 Zurich, Switzerland. Email: [email protected]
SUPPLEMENTARY INFORMATION
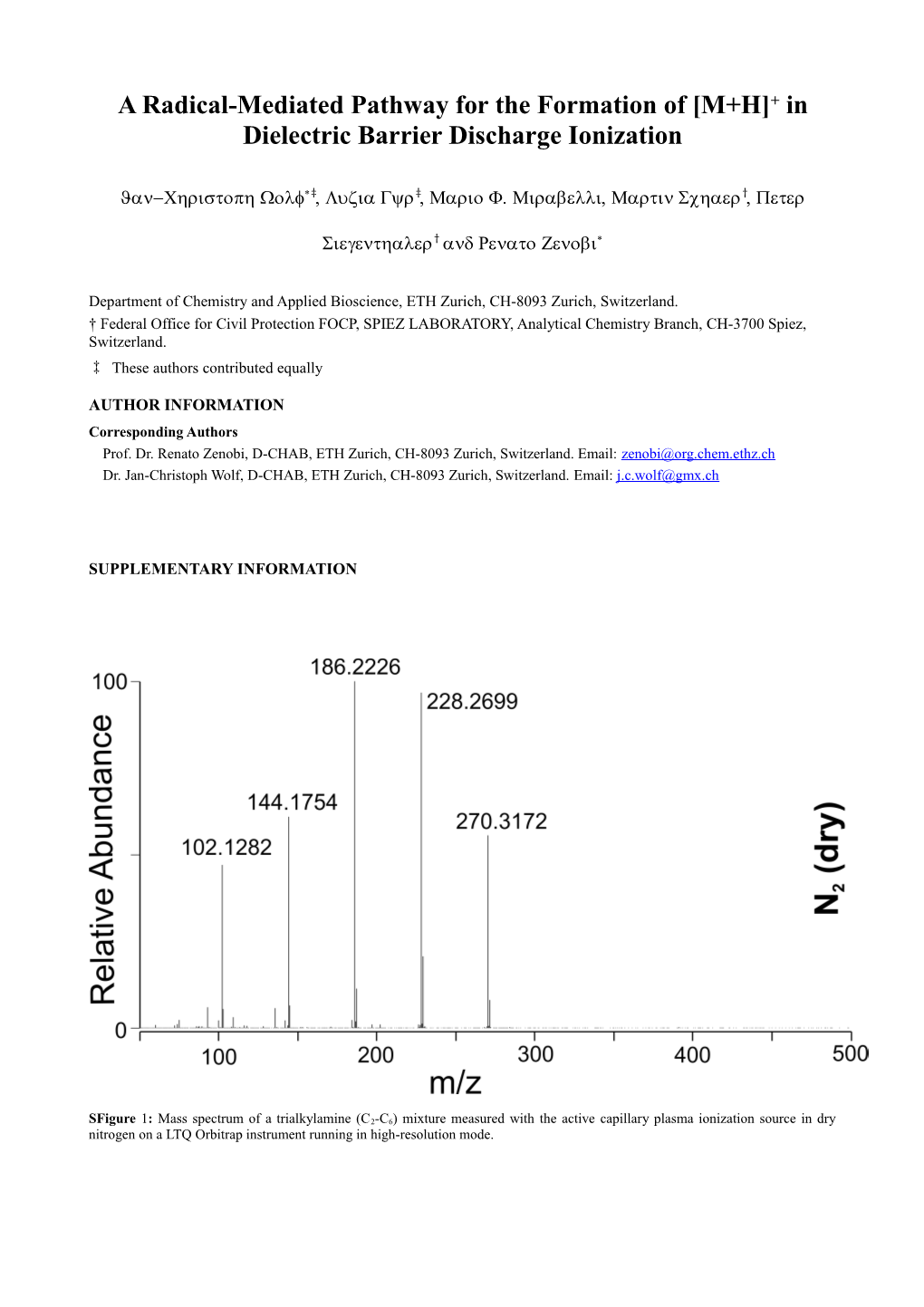
SFigure 1: Mass spectrum of a trialkylamine (C2-C6) mixture measured with the active capillary plasma ionization source in dry nitrogen on a LTQ Orbitrap instrument running in high-resolution mode. SFigure 2: Mass spectrum of a trialkylamine (C2-C6) mixture measured with the active capillary plasma ionization source in dry air on a Thermo LCQ Deca XP instrument.
SFigure 3: Mass spectrum of a trialkylamine (C2-C6) mixture measured with the active capillary plasma ionization source in dry CO2 on a Thermo LCQ Deca XP instrument. Note: some masses are not labeled, to facilitate depiction.
PAGE 2 2 SFigure 4: MS spectrum of tributylamine measured with the active capillary plasma ionization source in dry CO 2 on a Termo LCQ Deca XP instrument. 202 m/z was selected as precursor ion with an isolation window of 1 m/z.
2 SFigure 5: MS spectrum of a tripentylamine measured with the active capillary plasma ionization source in dry CO 2 on a Thermo LCQ Deca XP instrument. 244 m/z was selected as precursor ion with an isolation window of 1 m/z. 2 SFigure 6: MS spectrum of a trihexylamine measured with the active capillary plasma ionization source in dry CO 2 on a Thermo LCQ Deca XP instrument. 286 m/z was selected as precursor ion with an isolation window of 1 m/z.
SFigure 7: Proposed MS2 fragmentation pathway and corresponding fragment masse for the MOH+ ion of tributylamine.
PAGE 2 SFigure 8: Comparison of the molecular ion signal of tributylamine in dry air, infused together with CDCl3 a) and HPLC grade acetonitrile b). Spectra were recorded on a Thermo LCQ Deca XP instrument.
SFigure 9: Mass spectrum of a diethylethylphosphonate (DEEP) measured with the active capillary plasma ionization source in air (10% relative humidity) on a Thermo LCQ Deca XP instrument. SFigure 10: Mass spectrum of a diethylethylphosphonate (DEEP) measured with the active capillary plasma ionization source in air (70% relative humidity) on a Thermo LCQ Deca XP instrument.
+• SFigure 12: The free energy diagram of the oxidation reaction (eq. 7) of the tertiary amines with CO2 . R7 corresponds to the +• reactant of CO2 and M and the sum of the energies of the separated reactants was set to zero as common reference point. A7 means the adduct of these two reactants. TS7 and P7 corresponds to the transition state of the reaction 7 and to the products, respectively. PAGE 2 SFigure 13: The free energy diagram of the oxidation reaction (eq. 8) of the tertiary amines with CO2. R8 corresponds to the reactant +• of CO2 and M and the sum of the energies of the separated reactants was set to zero as common reference point. TS8 and P8 corresponds to the transition state of the reaction 8 and to the products, respectively.
+• SFigure 14: The free energy diagram of the hydride abstraction reaction (eq. 10) of the tertiary amines with CO2 . R10 corresponds +• to the reactant of CO2 and M and the sum of the energies of the separated reactants was set to zero as common reference point. A10, TS10 and P10 corresponds to the adduct of the two reactants, transition state and to the products of the reaction 10, respectively. SFigure 15: The free energy diagram of the hydride abstraction reaction (eq. 11) of the tertiary amines with CO2. R11 corresponds to +• the reactant of CO2 and M and the sum of the energies of the separated reactants was set to zero as common reference point. TS11 and P11 corresponds to the transition state and to the products of the reaction 11, respectively.
SFigure 16: The free energy diagram of the hydride abstraction reaction (eq. 12) of the oxidized tertiary amines. R12 corresponds to the reactant of [M+OH]+ and the sum of the energies of the separated reactants was set to zero as common reference point. TS12 and P12 corresponds to the transition state and to the products of the reaction 12, respectively.
PAGE 2 SFigure 17: The free energy diagram of the hydride abstraction reaction (eq. 13) of the protontated tertiary amines. R13 corresponds to the reactant of [M+H]+ and the sum of the energies of the separated reactants was set to zero as common reference point. TS13 and P13 corresponds to the transition state and to the products of the reaction 13, respectively.