Reaction Rates Study Guide Be sure to review all vocabulary and notes for Chapter 17
1. Describe how you would know that a reaction was taking place. What would you see?
2. Explain the collision theory. What is necessary for a chemical reaction to take place?
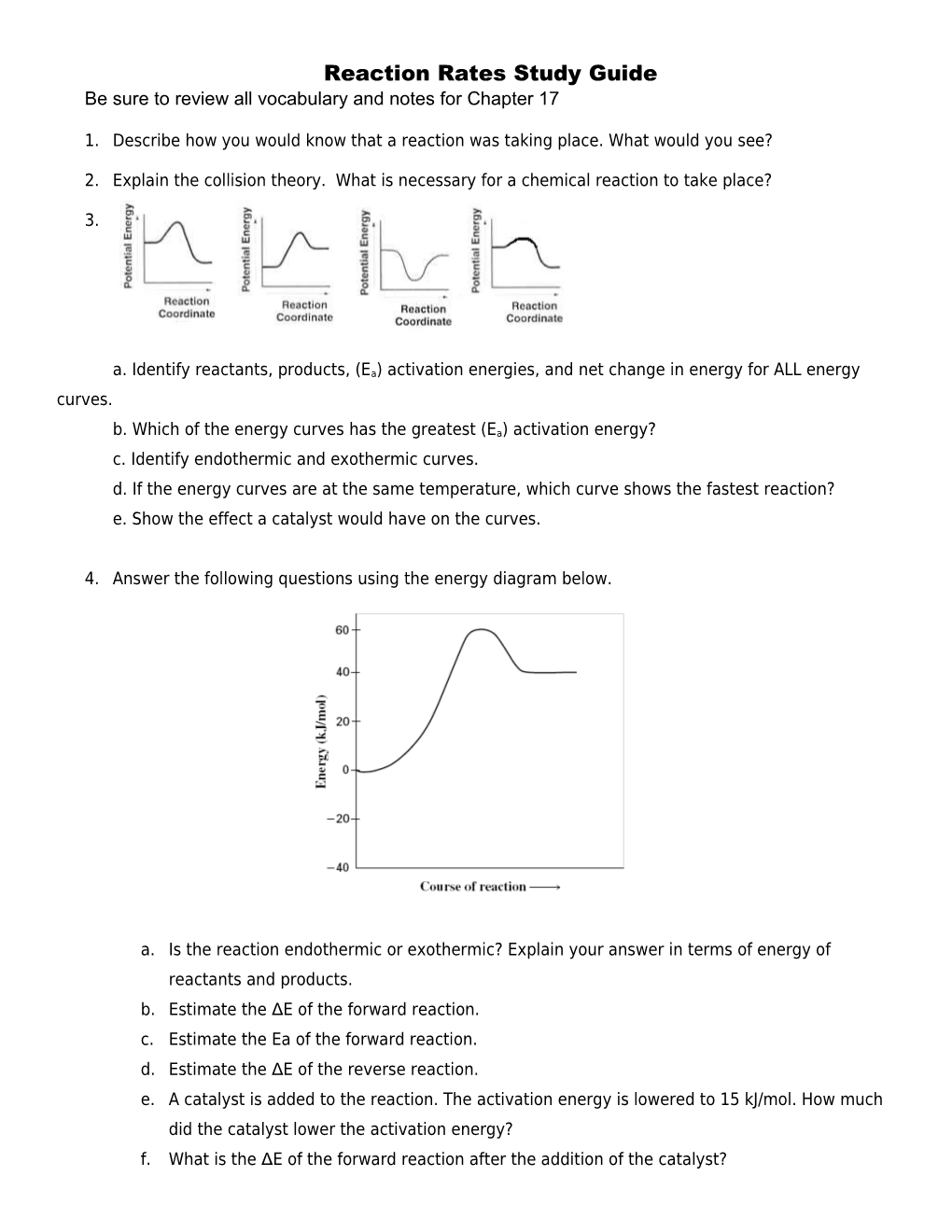
3.
a. Identify reactants, products, (Ea) activation energies, and net change in energy for ALL energy curves.
b. Which of the energy curves has the greatest (Ea) activation energy? c. Identify endothermic and exothermic curves. d. If the energy curves are at the same temperature, which curve shows the fastest reaction? e. Show the effect a catalyst would have on the curves.
4. Answer the following questions using the energy diagram below.
a. Is the reaction endothermic or exothermic? Explain your answer in terms of energy of reactants and products. b. Estimate the ΔE of the forward reaction. c. Estimate the Ea of the forward reaction. d. Estimate the ΔE of the reverse reaction. e. A catalyst is added to the reaction. The activation energy is lowered to 15 kJ/mol. How much did the catalyst lower the activation energy? f. What is the ΔE of the forward reaction after the addition of the catalyst? 5. List three factors that affect reaction rate and describe the effect they have.
Thermochemistry Review Worksheet
Be sure to review all vocabulary and notes for Chapter 16 and 17. Most of the multiple choice will be concept questions from these two things.
1. A laboratory volcano can be made from ammonia dichromate. When ignited, the compound decomposes into a fiery display. If the decomposition of 1mol ammonium dichromate produces 315kJ of heat energy at a constant pressure, how much heat energy would be produced by 28.3g of the solid? (NH4)2Cr2O7(s) N2(g) + 4H2O(g) + Cr2O3(s) 2. How many kilojoules of heat are evolved by a reaction in a calorimeter, where the temperature of 320.0g water increases from 19.5˚C to 22.83˚C? Given: The specific heat capacity of water is 4.186J/gK. 3. Suppose you pick up a 16Kg “shot-put” (ball of iron) at a track event. The iron ball has the same temperature as the atmosphere on a cool day, 16˚C. How many calories of heat energy must the iron ball absorb to reach the temperature of your body, 37˚C? Given: The specific heat capacity of iron is 0.460J/gK. 4. How many kilocalories of heat energy are required to heat all of the aluminum in a roll of aluminum foil, 500g, from room temperature25˚C to the temperature of a hot oven 250˚C? Given: The specific heat capacity of aluminum is 0.900J/gK. 5. Calculate the amount of heat released from the combustion of 35.0g of propane gas, C3H8. Given: The molar heat of combustion for propane is 2220kJ/mol. 6. Using the amount of heat released from the propane in problem five to warm up 200g of water, what would be the change in temperature of the water? The specific heat capacity of water is 4.186J/gK. 7. How much octane, C8H18, should go through combustion to produce 10,000kJ of energy? Given: The molar heat of combustion for octane is 5471kJ/mol. 8. When 5.8g of methane gas, CH4, reacts with oxygen gas to produce carbon dioxide gas and water vapor, 320kJ of energy is released. Calculate the molar enthalpy of combustion for methane in kJ/mol? 9. Calculate the total heat absorbed when 25 g of ice is raised from a -32 degrees Celsius to 127 degrees Celsius. (Hint: Draw a picture) 10. The reaction for the combustion of butane is … 2C2H10(g) + 13O2(g) 8CO2(g) + 10H2O(g) ΔHrxn = -4345kJ a. Is this reaction endothermic or exothermic? b. Calculate the number of grams of butane needed to produce 134.0J of energy.