CHM238-01 EXAM 2 October 14, 2002 103- points Name:
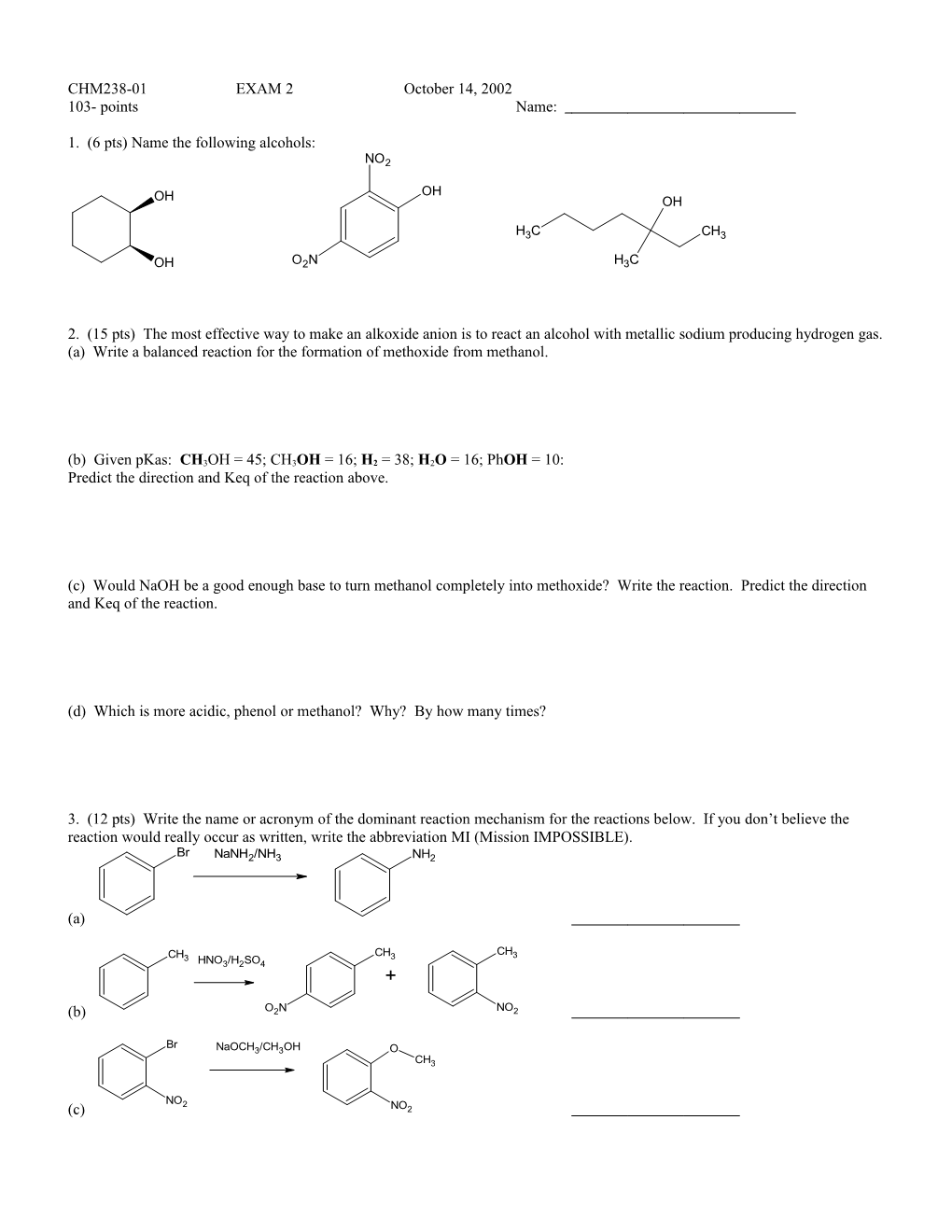
1. (6 pts) Name the following alcohols: NO2
OH OH OH
H3C CH3
OH O2N H3C
2. (15 pts) The most effective way to make an alkoxide anion is to react an alcohol with metallic sodium producing hydrogen gas. (a) Write a balanced reaction for the formation of methoxide from methanol.
(b) Given pKas: CH3OH = 45; CH3OH = 16; H2 = 38; H2O = 16; PhOH = 10: Predict the direction and Keq of the reaction above.
(c) Would NaOH be a good enough base to turn methanol completely into methoxide? Write the reaction. Predict the direction and Keq of the reaction.
(d) Which is more acidic, phenol or methanol? Why? By how many times?
3. (12 pts) Write the name or acronym of the dominant reaction mechanism for the reactions below. If you don’t believe the reaction would really occur as written, write the abbreviation MI (Mission IMPOSSIBLE). Br NaNH2/NH3 NH2
(a)
CH CH CH3 3 3 HNO3/H2SO4 +
(b) O2N NO2
Br NaOCH3/CH3OH O CH3
NO2 (c) NO2 O OH
LAH CH3 CH3
(d)
H3C
H3C OH concentrated aqueous acid + H2O CH2
CH3
(e)
OH Jones Reagent OH
O (f)
4. (12 pts) Choose the best set of reagents to carry out the following conversions to the highest yield form the choices given. Write the letter of the best choice in the blank. O
A. 1. HNO3, H2SO4 B. 1. Br2, FeBr3 C. 1. Cl , AlCl3 2. Br2, FeBr3 2. CH3Cl, AlCl3 2 Br2, FeBr3 + 3. NaOH (aq) 3. KMnO4, H3 O 3. H2 , Pt D. 1. (CH3)2CHCH2CH2Cl, AlCl3 E. 1. Br2, FeBr3 F. 1. CH3Cl, AlCl3 + 2. HNO3, H2SO4 2. HNO3, H2SO4 2. KMnO4, H3O 3. NaOH(aq) 3. Br2, FeBr3 O O O
G. 1. Cl , AlCl3 H. 1. Cl , AlCl3 I. 1. Cl , AlCl3 2. H2, Pt 2. H2, Pt 2 HNO3, H2SO4 3. HNO3, H2SO4 3. Br2, FeBr3 3. H2, Pt 0 J. 1. Br2, FeBr3 K. 1. Br2, FeBr3 L. 1. NaOH (aq), 350 C, high P 2. NaNH2, NH3 2. NaNH2, NH3 2. Br2, FeBr3 3. HNO3, H2SO4 3. HNO3, H2SO4
Br
(a)
O
Br OH
(b)
OH
(c) O2N
(d) O2N 5. (12 pts) Preparation of alcohols by Grignard reagents reacted with C=O compounds is very important. (a) If the Grignard reagent were phenyl Grignard, PhMgBr, draw the C=O compound would be the best one to use in order to make the following alcohols If it doesn’t work, write NR. (b) If the Grignard were, ethyl Grignard, EtMgBr, draw the C=O compound would be the best one to use in order to make the following alcohols. If it doesn’t work, write, NR. a = Carbonyl compound to use with Phenyl Grignard b = Carbonyl compound to use with Ethyl Grignard
HO
CH3
CH3
a= b= OH
CH3 a= b=
6. (18 pts) Provide the major organic products for the following reactions. If no reaction occurs, write NR. Do 6 out of 8. Cross out the one you don’t want graded or graded in order.
H3C Cl + AlCl3
H3C (a)
H3C NaOH (aq) 350C, high P
(b) Br
H2SO4 O + HNO3
(c) NH CH3
HO CrO3, H2SO4 (excess)
OH (d) H3C
HO PCC (excess)
OH (e) H3C CH2Cl2
O O 1. NaBH4
CH3 2. acid workup (f) H3C O
O O 1. LAH
CH3 2. acid workup (g) H3C O H3C CH3
NBS, FRI
(h)
7. (10 pts) Starting from benzene and any other organic or inorganic compounds, show routes to the following products. You will need at least two steps. Assume that you can separate o and p. OH
O
O2N OH
8. (10 pts) One problem with trying to react the following alcohol with HBr in order to get the alkyl bromide is the rearrangement that occurs under acidic conditions. Consider the situation shown below. (a) Provide arrows and necessary lone pairs to complete the reaction flow for the unexpected alcohol substitution reaction: H H H OH2 OH H Br H H C H O H C H2O 3 2 H C 3 CH3 H C H C 3 CH3 + + 3 3 CH3 CH3 CH3 H3C Br- H3C Br H3C Br- Br- H3C H3C
(b) What is the name of the starting alcohol?
(c) Which reagent(s) would you use if you wanted to substitute the alcohol with bromide in the same position and with inversion of configuration, without any rearrangement.
(d) This type of rearrangement can occur when trying to hydrate an alkene in acid. If we add water and acid (acid catalyzed hydration) to the 3-Methyl-1-butene, the main product is 2-methyl-2-butanol. HO H C 3 H2O H3C CH 2 CH3 + CH H 3 CH3 Which reagents would you use to make the starting alcohol in a above from 3-Methyl-1-butene, without rearrangement? 9. (10 pts) Identify the organic compound that matches most closely to the spectra on the page. Draw the structure and then draw lines from the H’s to the peaks in the NMR. Identify any peaks you can in the IR.