CHM 123 Extra Credit - Final Review Name Due on the FINAL EXAM DAY, no late assignment It is the students’ responsibility to review and study all other topics that were discussed in class. Do NOT assume the exam will only have these particular questions.
Chapter 10 Which drawing best accounts for the polarity of methanol, CH3OH, and the bond polarities that make a major contribution to the overall molecular polarity?
Answer: B
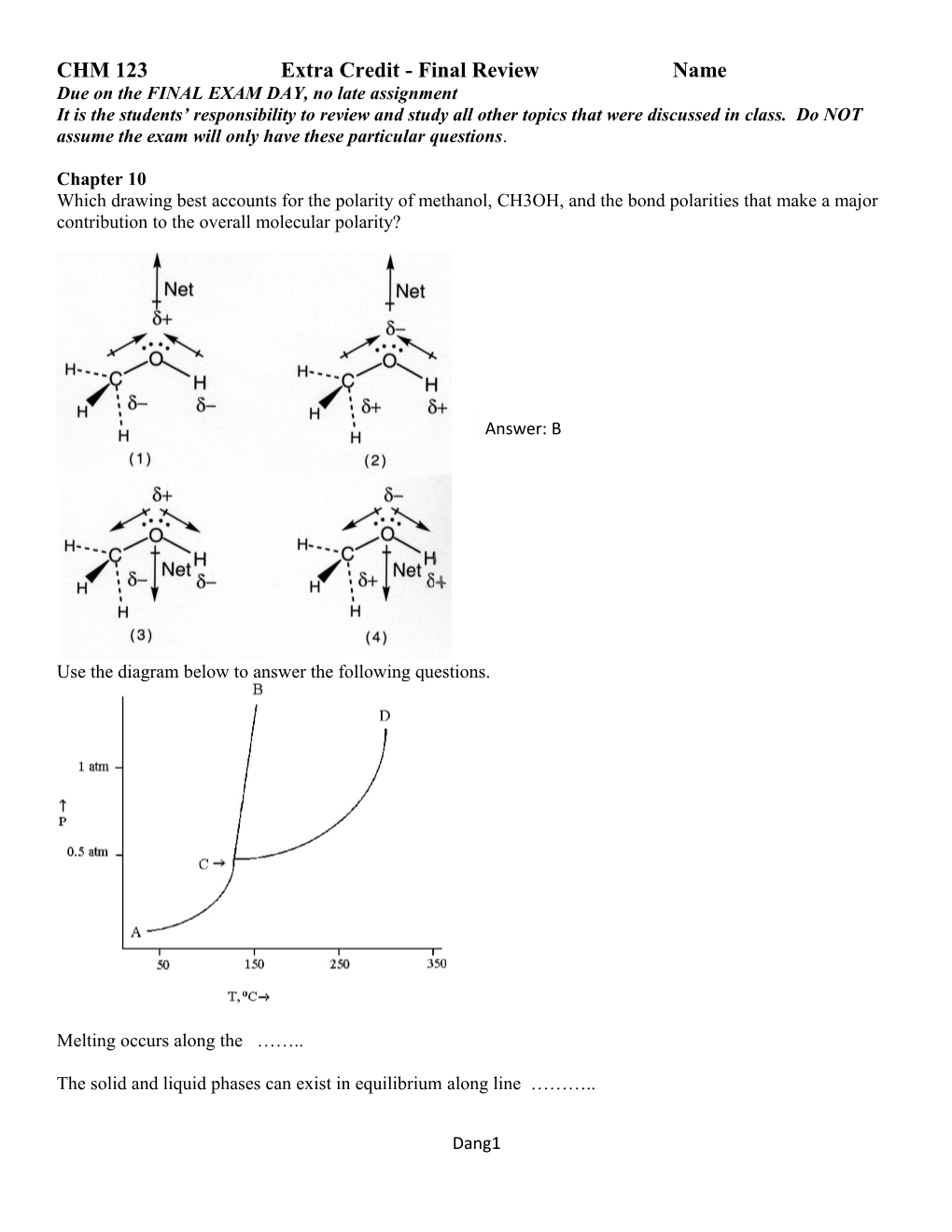
Use the diagram below to answer the following questions.
Melting occurs along the ……..
The solid and liquid phases can exist in equilibrium along line ………..
Dang1 The temperature and pressure at which all three phases can coexist in equilibrium is …….. From the phase diagram above, the minimum pressure at which this substance can exist in the liquid phase is ……..
The normal boiling point of this substance is approximately …..
What phases can be present at 200°C and 0.75 atm pressure?
What phase changes occur when the temperature is held constant at 140°C and the pressure is increased from 0.25 atm to 1.4 atm?
What phase changes occur when the pressure is held constant at 0.25 atm and the temperature increases from 100°C to 300°C?
Chapter 11
What is the mole fraction of ethanol in a solution made by dissolving 14.6 g of ethanol, C2H5OH, in 53.6 g of water? Answer: 0.0964
A solution is prepared by dissolving 17.75 g sulfuric acid, H2SO4, in enough water to make 100.0 mL of solution. If the density of the solution is 1.1094 g/mL, what is the molality? Answer: 1.940 m H2SO4
A solution is prepared by dissolving 17.75 g sulfuric acid, H2SO4, in enough water to make exactly 100.0 mL of solution. If the density of the solution is 1.1094 g/mL, what is the weight % H2SO4 in the solution? Answer: 16.00%
A solution is 2.25% by weight NaHCO3. How many grams of NaHCO3 are in 450.0 g of solution? Answer: 10.1 g
Dang2 The solubility of argon in water at 25°C is 0.0150 mol/L. What is the Henry's Law constant for argon if the partial pressure of argon in air is 0.00934 atm? Answer: 1.61 mol/(L ∙ atm)
Indicate how many particles are formed when the following solutes dissolve.
SOLUTE # OF PARTICLES SOLUTE # OF PARTICLES
sucrose (C12H22O11) magnesium chloride (MgCl2) sodium sulfate methanol (CH3OH) (Na2SO4)
When 5.0 g of CaCl2 dissolves in 50.0 g of water, what is the boiling point of the solution? (Kb Water = 0.512 oC/m)
The molal boiling point constant for ethyl alcohol is 1.22 oC/molal. Its boiling point is 78.4oC. A solution of 14.2 g of a nonvolatile nonelectrolyte in 264 g of the alcohol boils at 79.8oC. What is the molecular mass of the solute?
o 125 g of the non-volatile solute glucose, C6H12O6, is dissolved in 125 g of water at 25.0 C. IF the vapor pressure of water at 25.0oC is 23.7 Torr, what is the vapor pressure of the solution?
Calculate the Osmotic pressure at 50 degrees Celsius of a glucose solution C6H12O6 that has 60 grams of glucose dissolved in enough water to make 1500 ml.
Dang3 answer: 5.89 atm
Chapter 12 A concentration-time study of the gas phase reaction 2 A3 → 3 A2 produced the data in the table below. A concentration-time study of the gas phase reaction 2 A3 → 3 A2 produced the data in the table below Time (s) [A3] (M) [A2] (M) 0 4.00 × 10–4 0 10 2.00 × 10–4 3.00 × 10–4 20 1.00 × 10–4 4.50 × 10–4 30 5.00 × 10–4 ?
What is the average rate of formation of A2 in the time interval 20-30 seconds? Answer: 7.50 × 10–6 M/s
The following set of data was obtained by the method of initial rates for the reaction: (H3C)3CBr + OH- → (H3C)3COH + Br- What is the order of reaction with respect to ion, OH-?
[(H3C)3CBr] (M) [OH ] (M) Initial Rate (M/s) 0.25 0.25 1.1 × 10–4 0.50 0.25 2.2 × 10–4 0.50 0.50 2.2 × 10–4 Answer: zero
-5 -1 The first-order reaction, SO2Cl2 → SO2 + Cl2, has a rate constant equal to 2.20 × 10 s at 593 K. What percentage of the initial amount of SO2Cl2 will remain after 2.00 hours? Answer: 85.4%
Nitrogen dioxide decomposes at 300°C via a second-order process to produce nitrogen monoxide and oxygen according to the following chemical equation. 2 NO2(g) → 2 NO(g) + O2(g). A sample of NO2(g) is initially placed in a 2.50-L reaction vessel at 300°C. If the half-life and the rate constant -1 -1 at 300°C are 11 seconds and 0.54 M s , respectively, how many moles of NO2 were in the original sample? Dang4 Answer: 0.42 mol
The equation of tris(1, 10-phenanthroline)iron(II) in acid solution takes place according to the equation: 2+ 2+ + Fe(phen)3 + 3 H3O+ + 3 H2O → Fe(H2O)6 + 3 phenH If the activation energy is 126 kJ/mol and frequency factor is 8.62 × 1017 s-1, at what temperature is the rate constant equal to 3.63 × 10-3 s-1 for the first-order reaction? 50°C
The first-order isomerization reaction: cyclopropane → propene, has a rate constant of 1.10 × 10–4 s–1 at 470°C –4 –1 and 5.70 × 10 s at 500ºC. What is the activation energy, Ea, for the reaction?
260 kJ/mol
Chapter 15 What is the approximate pH at the equivalence point of a weak acid-strong base titration if 25 mL of aqueous hydrofluoric acid requires 30.00 mL of 0. 400 M NaOH? Ka = 6.76 × 10-4 for HF. 8.25
Dang5 What is the pH of the resulting solution if 45 mL of 0.432 M methylamine, CH3NH2, is added to 15 mL of -11 + 0.234 M HCl? Assume that the volumes of the solutions are additive. Ka = 2.70 × 10 for CH3NH3 . Answer: 11.23
Chapter 22 C has a decay constant, k = 1.209 × 10–4 yr–1 and a half-life of _____ years. Answer: 5.733 × 103
Neptunium-239 has a half-life of 2.35 days. How many days must elapse for a sample of 239Np to decay to 0.100% of its original quantity? 23.4 days
Dang6 If the age of the Earth is 4.5 billion years and the half-life of 40K is 1.26 billion years, what percent of the Earth's original amount of 40K remains today?
8.4%
Dang7