Conirostrum Cinereum) Andrea N
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
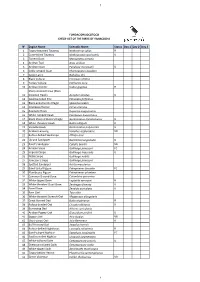
N° English Name Scientific Name Status Day 1
1 FUNDACIÓN JOCOTOCO CHECK-LIST OF THE BIRDS OF YANACOCHA N° English Name Scientific Name Status Day 1 Day 2 Day 3 1 Tawny-breasted Tinamou Nothocercus julius R 2 Curve-billed Tinamou Nothoprocta curvirostris U 3 Torrent Duck Merganetta armata 4 Andean Teal Anas andium 5 Andean Guan Penelope montagnii U 6 Sickle-winged Guan Chamaepetes goudotii 7 Cattle Egret Bubulcus ibis 8 Black Vulture Coragyps atratus 9 Turkey Vulture Cathartes aura 10 Andean Condor Vultur gryphus R Sharp-shinned Hawk (Plain- 11 breasted Hawk) Accipiter striatus U 12 Swallow-tailed Kite Elanoides forficatus 13 Black-and-chestnut Eagle Spizaetus isidori 14 Cinereous Harrier Circus cinereus 15 Roadside Hawk Rupornis magnirostris 16 White-rumped Hawk Parabuteo leucorrhous 17 Black-chested Buzzard-Eagle Geranoaetus melanoleucus U 18 White-throated Hawk Buteo albigula R 19 Variable Hawk Geranoaetus polyosoma U 20 Andean Lapwing Vanellus resplendens VR 21 Rufous-bellied Seedsnipe Attagis gayi 22 Upland Sandpiper Bartramia longicauda R 23 Baird's Sandpiper Calidris bairdii VR 24 Andean Snipe Gallinago jamesoni FC 25 Imperial Snipe Gallinago imperialis U 26 Noble Snipe Gallinago nobilis 27 Jameson's Snipe Gallinago jamesoni 28 Spotted Sandpiper Actitis macularius 29 Band-tailed Pigeon Patagoienas fasciata FC 30 Plumbeous Pigeon Patagioenas plumbea 31 Common Ground-Dove Columbina passerina 32 White-tipped Dove Leptotila verreauxi R 33 White-throated Quail-Dove Zentrygon frenata U 34 Eared Dove Zenaida auriculata U 35 Barn Owl Tyto alba 36 White-throated Screech-Owl Megascops -

Diversity and Natural History of Birds in Green Urban Areas of the City of Quito, Ecuador (America)
Diversity and natural history of birds in green urban areas of the city of Quito, Ecuador (America) Diego F. Cisneros-Heredia1,2,3* & Eliana Montenegro1 1 Universidad San Francisco de Quito, Colegio de Ciencias Biológicas y Ambientales, Laboratorio de Zoología Terrestre. Casilla Postal 17-1200-841, Quito, Ecuador 2 King’s College London, Department of Geography, Strand, London WC2R 2LS, UK 3 Aves&Conservación / BirdLife Ecuador, Quito, Ecuador * E-mail: [email protected] Abstract The diversity and ecology of urban bird communities have been extensively studied in Neartic and Paleartic areas, however, little is known about urban Neotropical areas. Quito, capital city of Ecuador, is located on a highland valley in the megadiverse tropical Andes. Founded in 1534, Quito did not increase significantly its urban area until the late 19th century, growing at an accelerated and unplanned rate during the 20th century. More than 100 species were known to inhabit in Quito at the end of the 19th century. Currently, most authors estimated that no more than 40 species occur in Quito, although no systematic bird studies have been conducted. Our research is a first approach to the avifauna of Quito, surveying the diversity living in green urban areas within the city borders. We used two field methodologies, i.e. line transects and point counts, to survey 16 green urban areas over 12 months. We recorded 65 species of birds, belonging to 20 families and 9 orders. Three species were the most common and frequent: Eared Dove Zenaida auriculata, Rufous-collared Sparrow Zonotrichia capensis, and Great Thrush Turdus fuscater, being omnivores and granivores adapted to anthropic habitats with low ecological complexity. -

A Case of Partial Albinism in Giant Conebill Oreomanes Fraseri
C o tin g a 1 8 A case of partial albinism in G iant C onebill O reom anes fraseri Grace P. Servat Cotinga 18 (2002): 80 Un individuo parcialmente albino del Pájaro de los Queñuales Oreomanes fraseri, fue observado y capturado en noviembre de 1997, en un bosque de Polylepis incana en la localidad de Japani, Lima, Perú. Este constituye el primer registro de albinismo para esta especie. Se presenta documentación fotográfica del individuo aberrante. Giant Conebill Oreomanes fraseri is a monotypic the Carnes Award from the American Ornitholo genus restricted to high-elevation Polylepis wood gists’ Union. lands, from south Colombia to south-west Bolivia3,5. Throughout the Andes of Peru O. fraseri is a con R e f e r e n c e s spicuous member of the highly threatened Polylepis 1. Arce, R. (1992) Distribución geográfica y avian community2. situación actual de los Polylepis en los I visited a Polylepis woodland in Japani (dpto. Departamentos de Ancash y Lima. Tesis. Lima, Huarochiri province, at 11°41'S 76°31'W and Universidad Nacional Agraria La Molina, 4140 m) from 28 November to 2 December 1997. A Lima. large (c. 300 ha) Polylepis incana woodland extends 2. Fjeldså, J. & Kessler, M. (1996) Conserving the along a chain of mountains, surrounded by bare ter biological diversity of Polylepis woodlands of rain, or dry shrubby vegetation1 (Fig. 1). On 30 the highlands of Peru and Bolivia. A contribu November, a partially albinistic O. fraseri was ob tion to sustainable natural resource manage served foraging on the bark of Polylepis trees, with ment in the high Andes. -

Dacninae Species Tree, Part I
Dacninae I: Nemosiini, Conirostrini, & Diglossini Hooded Tanager, Nemosia pileata Cherry-throated Tanager, Nemosia rourei Nemosiini Blue-backed Tanager, Cyanicterus cyanicterus White-capped Tanager, Sericossypha albocristata Scarlet-throated Tanager, Sericossypha loricata Bicolored Conebill, Conirostrum bicolor Pearly-breasted Conebill, Conirostrum margaritae Chestnut-vented Conebill, Conirostrum speciosum Conirostrini White-eared Conebill, Conirostrum leucogenys Capped Conebill, Conirostrum albifrons Giant Conebill, Conirostrum binghami Blue-backed Conebill, Conirostrum sitticolor White-browed Conebill, Conirostrum ferrugineiventre Tamarugo Conebill, Conirostrum tamarugense Rufous-browed Conebill, Conirostrum rufum Cinereous Conebill, Conirostrum cinereum Stripe-tailed Yellow-Finch, Pseudochloris citrina Gray-hooded Sierra Finch, Phrygilus gayi Patagonian Sierra Finch, Phrygilus patagonicus Peruvian Sierra Finch, Phrygilus punensis Black-hooded Sierra Finch, Phrygilus atriceps Gough Finch, Rowettia goughensis White-bridled Finch, Melanodera melanodera Yellow-bridled Finch, Melanodera xanthogramma Inaccessible Island Finch, Nesospiza acunhae Nightingale Island Finch, Nesospiza questi Wilkins’s Finch, Nesospiza wilkinsi Saffron Finch, Sicalis flaveola Grassland Yellow-Finch, Sicalis luteola Orange-fronted Yellow-Finch, Sicalis columbiana Sulphur-throated Finch, Sicalis taczanowskii Bright-rumped Yellow-Finch, Sicalis uropigyalis Citron-headed Yellow-Finch, Sicalis luteocephala Patagonian Yellow-Finch, Sicalis lebruni Greenish Yellow-Finch, -

The Natural Choice for Wildlife Holidays Welcome
HOLIDAYS WITH 100% FINANCIAL PROTECTION The natural choice for wildlife holidays Welcome After spending considerable time and effort reflecting, questioning what we do and how we do it, and scrutinising the processes within our office and the systems we use for support, I am delighted to say that we are imbued with a new vigour, undiminished enthusiasm, and greater optimism than ever. My own determination to continue building on the solid foundation of twenty years of experience in wildlife tourism, since we started from very humble beginnings – to offer what is simply the finest selection of high quality, good value, tailor-made wildlife holidays – remains undaunted, and is very much at the core of all we do. A physical move to high-tech office premises in the attractive city of Winchester leaves us much better connected to, and more closely integrated with, the outside world, and thus better able to receive visitors. Our team is leaner, tighter, more widely travelled and more knowledgeable than ever before, allowing us to focus on terrestrial, marine and – along with Dive Worldwide – submarine life without distraction. In planning this brochure we deliberately set out to whet the appetite, and make no mention of either dates or prices. As the vast majority of trips are tailored to our clients’ exact requirements – whether in terms of itinerary, duration, standard of accommodation or price – the itineraries herein serve merely as indications of what is possible. Thereafter, you can refine these suggestions in discussion with one of our experienced consultants to pin down your precise needs and wants, so we can together create the wildlife holiday of your dreams. -

Peru: from the Cusco Andes to the Manu
The critically endangered Royal Cinclodes - our bird-of-the-trip (all photos taken on this tour by Pete Morris) PERU: FROM THE CUSCO ANDES TO THE MANU 26 JULY – 12 AUGUST 2017 LEADERS: PETE MORRIS and GUNNAR ENGBLOM This brand new itinerary really was a tour of two halves! For the frst half of the tour we really were up on the roof of the world, exploring the Andes that surround Cusco up to altitudes in excess of 4000m. Cold clear air and fantastic snow-clad peaks were the order of the day here as we went about our task of seeking out a number of scarce, localized and seldom-seen endemics. For the second half of the tour we plunged down off of the mountains and took the long snaking Manu Road, right down to the Amazon basin. Here we traded the mountainous peaks for vistas of forest that stretched as far as the eye could see in one of the planet’s most diverse regions. Here, the temperatures rose in line with our ever growing list of sightings! In all, we amassed a grand total of 537 species of birds, including 36 which provided audio encounters only! As we all know though, it’s not necessarily the shear number of species that counts, but more the quality, and we found many high quality species. New species for the Birdquest life list included Apurimac Spinetail, Vilcabamba Thistletail, Am- pay (still to be described) and Vilcabamba Tapaculos and Apurimac Brushfnch, whilst other montane goodies included the stunning Bearded Mountaineer, White-tufted Sunbeam the critically endangered Royal Cinclodes, 1 BirdQuest Tour Report: Peru: From the Cusco Andes to The Manu 2017 www.birdquest-tours.com These wonderful Blue-headed Macaws were a brilliant highlight near to Atalaya. -

21 Sep 2018 Lists of Victims and Hosts of the Parasitic
version: 21 Sep 2018 Lists of victims and hosts of the parasitic cowbirds (Molothrus). Peter E. Lowther, Field Museum Brood parasitism is an awkward term to describe an interaction between two species in which, as in predator-prey relationships, one species gains at the expense of the other. Brood parasites "prey" upon parental care. Victimized species usually have reduced breeding success, partly because of the additional cost of caring for alien eggs and young, and partly because of the behavior of brood parasites (both adults and young) which may directly and adversely affect the survival of the victim's own eggs or young. About 1% of all bird species, among 7 families, are brood parasites. The 5 species of brood parasitic “cowbirds” are currently all treated as members of the genus Molothrus. Host selection is an active process. Not all species co-occurring with brood parasites are equally likely to be selected nor are they of equal quality as hosts. Rather, to varying degrees, brood parasites are specialized for certain categories of hosts. Brood parasites may rely on a single host species to rear their young or may distribute their eggs among many species, seemingly without regard to any characteristics of potential hosts. Lists of species are not the best means to describe interactions between a brood parasitic species and its hosts. Such lists do not necessarily reflect the taxonomy used by the brood parasites themselves nor do they accurately reflect the complex interactions within bird communities (see Ortega 1998: 183-184). Host lists do, however, offer some insight into the process of host selection and do emphasize the wide variety of features than can impact on host selection. -

FIELD GUIDES BIRDING TOURS: Colombia: Bogota, the Magdalena
Field Guides Tour Report Colombia: Bogota, the Magdalena Valley, and Santa Marta 2014 Jan 11, 2014 to Jan 27, 2014 Jesse Fagan & Trevor Ellery For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. A fun group and the most productive tour we have had to date! We observed 582 bird taxa in 17 days of birding, which beat our record last year of 555 by a bunch. As we fine-tune our birding route and learn more about Colombian birds things just seem to get better and better. This year we saw 33 endemics and loads of interesting subspecies and near-endemics. Highlights included a female Blue- billed Curassow, Kelp Gull(s) at Los Camerones (only the second time it has been recorded in Colombia), Dwarf and Pavonine cuckoos (the latter a lifer for Trevor!), a splendid Crested Owl, Sapphire- bellied Hummingbird (nice comparisons with Sapphire-throated), Double-banded Graytail in the coffee finca below Reinita Cielo Azul lodge, the always elusive Santa Marta Bush-Tyrant and antpitta, Turquoise Dacnis, and singing Yellow-bellied Siskin. It is really hard to pick just one from so many! I want to thank all of you again for a really enjoyable trip. Thanks also to Trevor Ellery, our local guide, and Giovanni, our driver, for their hard work. I look forward to seeing you again in the field. Bird On. --Jesse a.k.a. Motmot (from Lima, Peru) KEYS FOR THIS LIST One of the following keys may be shown in brackets for individual species as appropriate: * = heard only, I = introduced, E = endemic, N = nesting, a = austral migrant, b = boreal migrant This dazzling Black-cheeked Mountain-Tanager is a Santa Marta endemic; it was one of 33 endemics we tallied on this species-rich tour. -

Santiago Merino Rodríguez Generated From: Editor CVN De FECYT Date of Document: 19/01/2021 V 1.4.3 Ed0a4acb17f1df7c4a134e698ca14615
Santiago Merino Rodríguez Generated from: Editor CVN de FECYT Date of document: 19/01/2021 v 1.4.3 ed0a4acb17f1df7c4a134e698ca14615 This electronic file (PDF) has embedded CVN technology (CVN-XML). The CVN technology of this file allows you to export and import curricular data from and to any compatible data base. List of adapted databases available at: http://cvn.fecyt.es/ ed0a4acb17f1df7c4a134e698ca14615 Summary of CV This section describes briefly a summary of your career in science, academic and research; the main scientific and technological achievements and goals in your line of research in the medium -and long- term. It also includes other important aspects or peculiarities. My line of research has been always linked to the study of the host-parasite interactions. These studies not only have focused on the effects of parasites on the fitness of their hosts and therefore its value as a selective force in nature, but also on the evolutionary strategies of transmission of different species of parasites. Throughout my career I have studied various host-parasite systems in populations of wild animals including mammals, reptiles and mostly birds, as well as their ectoparasites and endoparasites, mainly blood parasites. This has allowed me to obtain an overview about the particularities of different systems under study, which include very different life cycles. The team that I lead has always been characterized by used experimental approaches for the study of the host-parasite relationship in wild populations. We have been pioneers in the study of some variables of interest in these populations, such as heat stress proteins as parasitic stress indicators, and we have been pioneers in experimental studies of parasite load modification in blood parasites in wild populations. -

An Analysis of Physical, Physiological, and Optical Aspects of Avian Coloration with Emphasis on Wood-Warblers
(ISBN: 0-943610-47-8) AN ANALYSIS OF PHYSICAL, PHYSIOLOGICAL, AND OPTICAL ASPECTS OF AVIAN COLORATION WITH EMPHASIS ON WOOD-WARBLERS BY EDWARD H. BURTT, JR. Department of Zoology Ohio Wesleyan University Delaware, Ohio 43015 ORNITHOLOGICAL MONOGRAPHS NO. 38 PUBLISHED BY THE AMERICAN ORNITHOLOGISTS' UNION WASHINGTON, D.C. 1986 AN ANALYSIS OF PHYSICAL, PHYSIOLOGICAL, AND OPTICAL ASPECTS OF AVIAN COLORATION WITH EMPHASIS ON WOOD-WARBLERS ORNITHOLOGICAL MONOGRAPHS This series,published by the American Ornithologists' Union, has been estab- lished for major papers too long for inclusion in the Union's journal, The Auk. Publication has been made possiblethrough the generosityof the late Mrs. Carl Tucker and the Marcia Brady Tucker Foundation, Inc. Correspondenceconcerning manuscripts for publication in the seriesshould be addressedto the Editor, Dr. David W. Johnston,Department of Biology, George Mason University, Fairfax, VA 22030. Copies of Ornithological Monographs may be ordered from the Assistant to the Treasurer of the AOU, Frank R. Moore, Department of Biology, University of Southern Mississippi, Southern Station Box 5018, Hattiesburg, Mississippi 39406. (See price list on back and inside back covers.) Ornithological Monographs, No. 38, x + 126 pp. Editors of OrnithologicalMonographs, David W. Johnstonand Mercedes S. Foster Special Reviewers for this issue, Sievert A. Rohwer, Department of Zo- ology, University of Washington, Seattle, Washington; William J. Hamilton III, Division of Environmental Studies, University of Cal- ifornia, Davis, California Author, Edward H. Burtt, Jr., Department of Zoology, Ohio Wesleyan University, Delaware, Ohio 43015 First received, 24 October 1982; accepted 11 March 1983; final revision completed 9 April 1985 Issued May 1, 1986 Price $15.00 prepaid ($12.50 to AOU members). -

Huacarpay – Acjanacu- Pillahuata – San Pedro
Birding program – Manu National Park 08 days private service Day 01: Cusco – Huacarpay – Acjanacu- Pillahuata – San Pedro We depart from Cusco around 04:30 a.m. arriving at Lake Huarcarpay 30 minutes later. Here we take time and spend the entire day observing highland lake and marsh birds. The best is to follow the road that goes around the lake and start seeing most of the waterfowl typical of Andean wetlands including grebes, ducks, gallinules and coots. The wet grasslands in front of the marsh are good for shorebirds and Andean Negrito. We can look for occasional migrants such as the Hudsonian Godwit (rare) and Baird’s Sandpiper. The many- Coloured Rush-Tyrant and two endemic, the Rusty-fronted Canastero that can be seen around the ruins on the hillside above the Lake and a spectacular hummingbird, the Bearded Mountaineer. We continue to the interesting tombs of Ninamarca, commonly known as “Chullpas”. We continue to Paucartambo, a picturesque Spanish colonial town and walk the surrounding area and semi-humid montane scrub where a Taczanowski’s Tinamou has been reported here. Common to see around are: White-rufted Sunbeam, Creamy-crested Spintail, Cinereous Conebill, Torrent Duck. Our vehicle wll take us up to Acjanacu pass with its elfin forest marks the beginning of the Cultural Zone of Manu Biosphere Reserve. Birders stay the night here because it is possible to see White-browed chat-tyrant, the Chestnut-breasted mountain-finch, Puna Thistle tail, Paramo Pipit, Diademed Tapaculo, Puna Tapaculo, Masked flowerpiercer, Golden-colared Tanager, Drab hemispingus and small mixed flocks with among others. -

The Feeding Ecology of Tanagers and Honeycreepers in Trinidad
THE FEEDING ECOLOGY OF TANAGERS AND HONEYCREEPERS IN TRINIDAD B^•B^•^ K. S•ow ^•r• D. W. S•ow Dtmi•G the last 131/2months of our 41/2-years' residence in Trinidad (August 1960 to September 1961) we kept systematicrecords of the feedingbehavior of the commonertanagers and honeycreepers.By this time, besidesknowing the bird specieswell, we had learned to identify most of the trees and shrubs,particularly those in the northern mountain range where we lived. The correct systematictreatment of the tanagersand honeycreepersis still uncertain. Formerly they were separated as Thraupidae and Coerebidae,but Beecher (1951) argued that the Coerebidaeare a hetero- geneousgroup and recommendedplacing Coereba and Conirostrumwith the Parulidaeand the otherswith the Thraupidae. Whatevertheir correct systematicarrangement, they form a natural ecologicalgroup of small to medium-sized birds of wooded habitats that take a mixed diet of insects and fruit, with somenectar. The tanagersare structurally unspecialized as a group, but the honeycreepersin beak and tongue are to some extent specializedfor nectar-eating. As fruit-eaters,both tanagersand honey- creeperstypically exploit the smaller, succulent fruits of trees, shrubs, and vines,and are ecologicallyquite distinct from the larger, specialized fruit-eating birds that exploit the larger and more nutritious fruits of palms,Lauraceae, Burseraceae, and someother tree families (Snow, 1971). They are commonand conspicuousbirds of the neotropicalforests, where many speciescoexist, frequently with little obviousecological