ICD-10 Second Edition Volume 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hysteroscopy with Dilation and Curretage (D & C)
501 19th Street, Trustees Tower FORT SANDERS WOMEN’S SPECIALISTS 1924 Pinnacle Point Way Suite 401, Knoxville Tn 37916 P# 865-331-1122 F# 865-331-1976 Suite 200, Knoxville Tn 37922 Dr. Curtis Elam, M.D., FACOG, AIMIS, Dr. David Owen, M.D., FACOG, Dr. Brooke Foulk, M.D., FACOG Dr. Dean Turner M.D., FACOG, ASCCP, Dr. F. Robert McKeown III, M.D., FACOG, AIMIS, Dr. Steven Pierce M.D., Dr. G. Walton Smith, M.D., FACOG, Dr. Susan Robertson, M.D., FACOG HYSTEROSCOPY WITH DILATION AND CURRETAGE (D & C) Please read and sign the following consent form when you feel that you completely understand the surgical procedure that is to be performed and after you have asked all of your questions. If you have any further questions or concerns, please contact our office prior to your procedure so that we may clarify any pertinent issues. Definition: HysterosCopy is an outpatient procedure that allows your doctor direct visualization of the inside of the uterine cavity (womb) by inserting a thin lighted telescope (hysteroscope) through the vagina (birth canal) and cervix, without making an abdominal incision. This procedure enables your doctor to examine the lining of the uterus, look for polyps, fibroids, scar tissue, blockages of the fallopian tubes, and abnormal partitions. In addition, this procedure allows your doctor to remove or surgically treat many of the abnormalities seen. Dilation and Curettage (D&C) allows your doctor to take a sample of the tissue that lines your uterus (endometrium) and/or to remove polyps, fibroid tumors, or hyperplasia. Suction D&C is used in cases of miscarriage. -

A Discursive Approach to Female Circumcision: Why the United Nations Should Drop the One-Sided Conversation in Favor of the Vagina Dialogues
NORTH CAROLINA JOURNAL OF INTERNATIONAL LAW Volume 38 Number 2 Article 6 Winter 2013 A Discursive Approach to Female Circumcision: Why the United Nations Should Drop the One-Sided Conversation in Favor of the Vagina Dialogues Kathleen Bradshaw Follow this and additional works at: https://scholarship.law.unc.edu/ncilj Recommended Citation Kathleen Bradshaw, A Discursive Approach to Female Circumcision: Why the United Nations Should Drop the One-Sided Conversation in Favor of the Vagina Dialogues, 38 N.C. J. INT'L L. 601 (2012). Available at: https://scholarship.law.unc.edu/ncilj/vol38/iss2/6 This Note is brought to you for free and open access by Carolina Law Scholarship Repository. It has been accepted for inclusion in North Carolina Journal of International Law by an authorized editor of Carolina Law Scholarship Repository. For more information, please contact [email protected]. A Discursive Approach to Female Circumcision: Why the United Nations Should Drop the One-Sided Conversation in Favor of the Vagina Dialogues Cover Page Footnote International Law; Commercial Law; Law This note is available in North Carolina Journal of International Law: https://scholarship.law.unc.edu/ncilj/vol38/iss2/ 6 A Discursive Approach to Female Circumcision: Why the United Nations Should Drop the One-Sided Conversation in Favor of the Vagina Dialogues KATHLEEN BRADSHAWt I. Introduction ........................................602 II. Background................................ 608 A. Female Circumcision ...................... 608 B. International Legal Response....................610 III. Discussion......................... ........ 613 A. Foreign Domestic Legislation............. ... .......... 616 B. Enforcement.. ...................... ...... 617 C. Cultural Insensitivity: Bad for Development..............620 1. Human Rights, Culture, and Development: The United Nations ................... ............... 621 2. -

Urology 1 Cystoscopes
Urology 1 Cystoscopes 2 Urethrotomes 3 Resectoscopes 4 Uretero-Renoscopes 5 Nephroscopes 6 Lithotripsy (UreTron) 7 Laser Therapy 8 Small Caliber 9 Fluid Management 10 Accessories Richard Wolf Medical Instruments Corporation assumes no responsibility or liability for any errors or omissions in the content of this catalog. The information contained in this catalog is provided on an “as is” basis with no guarantees of completeness, accuracy, usefulness, or timeliness, and without warranties of any kind whatsoever, expressed or implied. 1777- 02.01-1118USA Cysto-Urethroscopes 8650 E-line design CYSTOSCOPES The E-line design guarantees optimum handling and safe, fatigue-free operation as well as a wide range of possible combinations. Basic Set for Cystoscopy Cysto-urethroscope sheath, 19.5 Fr. with obturator 8650.0341 Adapter with 1 instrument port 8650.264 Insert with Albarran deflector, 2 instrument ports 8650.204 Sterile universal sealing valve (pack of 5) 4712348 Viewing obturator 8650.724 Biopsy forceps Marburg 8650.614 Grasping forceps 8650.684 PANOVIEW telescope, 0° 8650.414 PANOVIEW telescope, 70° 8650.415 Otis urethrotome 8517.00 822.31 822.13 822.31 Flexible connector 822.13 Tray 8585030 1777- 02.01-1118USA 2 PANOVIEW Telescopes Overview CYSTOSCOPES Ø Viewing direction Color code Application mm 0° blue Standard 4 8650.414 12° orange Standard 4 8654.431 Standard 4 8654.422 30° red Long sheath 4 8668.433* 70° yellow Standard 4 8650.415 * Only 25° available. 1777- 02.01-1118USA 3 Cysto-Urethroscope 8650 for telescope 4 mm, 0°, 12°, 30°, 70° and adapters CYSTOSCOPES Sheaths and obturators Adapters Sheath incl. -

Physical Therapy, Occupational Therapy, and Speech and Language Pathology Providers
PhysicalPhysical Therapy,Therapy, OccupationalOccupational Therapy,Therapy, andand SpeechSpeech andand LanguageLanguage PathologyPathology ServicesServices ARCHIVAL USE ONLY Refer to the Online Handbook for current policy CContacting Wisconsin Medicaid Web Site dhfs.wisconsin.gov/ The Web site contains information for providers and recipients about the Available 24 hours a day, seven days a week following: • Program requirements. • Maximum allowable fee schedules. • Publications. • Professional relations representatives. • Forms. • Certification packets. Automated Voice Response System (800) 947-3544 (608) 221-4247 The Automated Voice Response system provides computerized voice Available 24 hours a day, seven days a week responses about the following: • Recipient eligibility. • Claim status. • Prior authorization (PA) status. • Checkwrite information. Provider Services (800) 947-9627 (608) 221-9883 Correspondents assist providers with questions about the following: Available: • Clarification of program ARCHIVAL• Resolving claim denials. USE ONLY8:30 a.m. - 4:30 p.m. (M, W-F) requirements. • Provider certification. 9:30 a.m. - 4:30 p.m. (T) • Recipient eligibility. Refer to the Online HandbookAvailable for pharmacy services: 8:30 a.m. - 6:00 p.m. (M, W-F) for current policy9:30 a.m. - 6:00 p.m. (T) Division of Health Care Financing (608) 221-9036 Electronic Data Interchange Helpdesk e-mail: [email protected] Correspondents assist providers with technical questions about the following: Available 8:30 a.m. - 4:30 p.m. (M-F) • Electronic transactions. • Provider Electronic Solutions • Companion documents. software. Web Prior Authorization Technical Helpdesk (608) 221-9730 Correspondents assist providers with Web PA-related technical questions Available 8:30 a.m. - 4:30 p.m. (M-F) about the following: • User registration. -
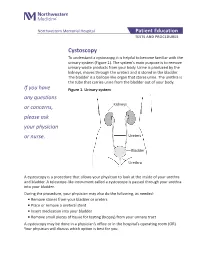
Cystoscopy to Understand a Cystoscopy, It Is Helpful to Become Familiar with the Urinary System (Figure 1)
Northwestern Memorial Hospital Patient Education TESTS AND PROCEDURES Cystoscopy To understand a cystoscopy, it is helpful to become familiar with the urinary system (Figure 1). The system’s main purpose is to remove urinary waste products from your body. Urine is produced by the kidneys, moves through the ureters and is stored in the bladder. The bladder is a balloon-like organ that stores urine. The urethra is the tube that carries urine from the bladder out of your body. If you have Figure 1. Urinary system any questions or concerns, Kidneys please ask your physician or nurse. Ureters Bladder Urethra A cystoscopy is a procedure that allows your physician to look at the inside of your urethra and bladder. A telescope-like instrument called a cystoscope is passed through your urethra into your bladder. During the procedure, your physician may also do the following, as needed: ■ Remove stones from your bladder or ureters ■ Place or remove a ureteral stent ■ Insert medication into your bladder ■ Remove small pieces of tissue for testing (biopsy) from your urinary tract A cystoscopy may be done in a physician’s office or in the hospital’s operating room (OR). Your physician will discuss which option is best for you. Preparation and procedure If the test is done in the OR, you will be asked to sign a written consent. The OR procedure and any special preparation will be explained to you. There may be some discomfort during the examination. Some patients may require sedation or anesthesia. Depending on the type of medication used for your procedure, you will be told if you need to stop eating and drinking before your procedure. -

Utility of the Digital Rectal Examination in the Emergency Department: a Review
The Journal of Emergency Medicine, Vol. 43, No. 6, pp. 1196–1204, 2012 Published by Elsevier Inc. Printed in the USA 0736-4679/$ - see front matter http://dx.doi.org/10.1016/j.jemermed.2012.06.015 Clinical Reviews UTILITY OF THE DIGITAL RECTAL EXAMINATION IN THE EMERGENCY DEPARTMENT: A REVIEW Chad Kessler, MD, MHPE*† and Stephen J. Bauer, MD† *Department of Emergency Medicine, Jesse Brown VA Medical Center and †University of Illinois-Chicago College of Medicine, Chicago, Illinois Reprint Address: Chad Kessler, MD, MHPE, Department of Emergency Medicine, Jesse Brown Veterans Hospital, 820 S Damen Ave., M/C 111, Chicago, IL 60612 , Abstract—Background: The digital rectal examination abdominal pain and acute appendicitis. Stool obtained by (DRE) has been reflexively performed to evaluate common DRE doesn’t seem to increase the false-positive rate of chief complaints in the Emergency Department without FOBTs, and the DRE correlated moderately well with anal knowing its true utility in diagnosis. Objective: Medical lit- manometric measurements in determining anal sphincter erature databases were searched for the most relevant arti- tone. Published by Elsevier Inc. cles pertaining to: the utility of the DRE in evaluating abdominal pain and acute appendicitis, the false-positive , Keywords—digital rectal; utility; review; Emergency rate of fecal occult blood tests (FOBT) from stool obtained Department; evidence-based medicine by DRE or spontaneous passage, and the correlation be- tween DRE and anal manometry in determining anal tone. Discussion: Sixteen articles met our inclusion criteria; there INTRODUCTION were two for abdominal pain, five for appendicitis, six for anal tone, and three for fecal occult blood. -

An Update on Dual-Energy X-Ray Absorptiometry Glen M
An Update on Dual-Energy X-Ray Absorptiometry Glen M. Blake, PhD, and Ignac Fogelman, MD Dual-energy x-ray absorptiometry (DXA) scans to measure bone mineral density at the spine and hip have an important role in the evaluation of individuals at risk of osteoporosis, and in helping clinicians advise patients about the appropriate use of antifracture treat- ment. Compared with alternative bone densitometry techniques, hip and spine DXA exam- inations have several advantages that include a consensus that bone mineral density results should be interpreted using the World Health Organization T score definition of osteoporosis, a proven ability to predict fracture risk, proven effectiveness at targeting antifracture therapies, and the ability to monitor response to treatment. This review dis- cusses the evidence for these and other clinical aspects of DXA scanning. Particular attention is directed at the new World Health Organization Fracture Risk Assessment Tool (FRAX) algorithm, which uses clinical risk factors in addition to a hip DXA scan to predict a patient’s 10-year probability of suffering an osteoporotic fracture. We also discuss the recently published clinical guidelines that incorporate the FRAX fracture risk assessment in decisions about patient treatment. Semin Nucl Med 40:62-73 © 2010 Elsevier Inc. All rights reserved. steoporosis is widely recognized as an important public porosis before fractures occur and the development of effec- Ohealth problem because of the significant morbidity, tive treatments. Measurements of bone mineral -

The Differences Between ICD-9 and ICD-10
Preparing for the ICD-10 Code Set: Fact Sheet 2 October 1, 2015 Compliance Date Get the Facts to be Compliant Alert: The new ICD-10 compliance date is October 1, 2015. The Differences Between ICD-9 and ICD-10 This is the second fact sheet in a series and is focused on the differences between the ICD-9 and ICD-10 code sets. Collectively, the fact sheets will provide information, guidance, and checklists to assist you with understanding what you need to do to implement the ICD-10 code set. The ICD-10 code sets are not a simple update of the ICD-9 code set. The ICD-10 code sets have fundamental changes in structure and concepts that make them very different from ICD-9. Because of these differences, it is important to develop a preliminary understanding of the changes from ICD-9 to ICD-10. This basic understanding of the differences will then identify more detailed training that will be needed to appropriately use the ICD-10 code sets. In addition, seeing the differences between the code sets will raise awareness of the complexities of converting to the ICD-10 codes. Overall Comparisons of ICD-9 to ICD-10 Issues today with the ICD-9 diagnosis and procedure code sets are addressed in ICD-10. One concern today with ICD-9 is the lack of specificity of the information conveyed in the codes. For example, if a patient is seen for treatment of a burn on the right arm, the ICD-9 diagnosis code does not distinguish that the burn is on the right arm. -

Description of Alternative Approaches to Measure and Place a Value on Hospital Products in Seven Oecd Countries
OECD Health Working Papers No. 56 Description of Alternative Approaches to Measure Luca Lorenzoni, and Place a Value Mark Pearson on Hospital Products in Seven OECD Countries https://dx.doi.org/10.1787/5kgdt91bpq24-en Unclassified DELSA/HEA/WD/HWP(2011)2 Organisation de Coopération et de Développement Économiques Organisation for Economic Co-operation and Development 14-Apr-2011 ___________________________________________________________________________________________ _____________ English text only DIRECTORATE FOR EMPLOYMENT, LABOUR AND SOCIAL AFFAIRS HEALTH COMMITTEE Unclassified DELSA/HEA/WD/HWP(2011)2 Health Working Papers OECD HEALTH WORKING PAPERS NO. 56 DESCRIPTION OF ALTERNATIVE APPROACHES TO MEASURE AND PLACE A VALUE ON HOSPITAL PRODUCTS IN SEVEN OECD COUNTRIES Luca Lorenzoni and Mark Pearson JEL Classification: H51, I12, and I19 English text only JT03300281 Document complet disponible sur OLIS dans son format d'origine Complete document available on OLIS in its original format DELSA/HEA/WD/HWP(2011)2 DIRECTORATE FOR EMPLOYMENT, LABOUR AND SOCIAL AFFAIRS www.oecd.org/els OECD HEALTH WORKING PAPERS http://www.oecd.org/els/health/workingpapers This series is designed to make available to a wider readership health studies prepared for use within the OECD. Authorship is usually collective, but principal writers are named. The papers are generally available only in their original language – English or French – with a summary in the other. Comment on the series is welcome, and should be sent to the Directorate for Employment, Labour and Social Affairs, 2, rue André-Pascal, 75775 PARIS CEDEX 16, France. The opinions expressed and arguments employed here are the responsibility of the author(s) and do not necessarily reflect those of the OECD. -

Helping Physicians Succeed with ICD-10-CM January 24, 2014
Helping Physicians Succeed with ICD-10-CM January 24, 2014 Paul Belton, Vice President Corporate Compliance Agenda • Executive Summary • Clinical Roots of ICD-10 • Why physicians should care • How ICD-10 will benefit physicians • Taking control of ICD-10 • Personal Learning Experiences and Goals • ICD-10 Resources 2 Last Call for ICD-9-CM October 1, 2013 could be a day sentimental HIM Veterans raise a glass to a longtime friend – or perhaps foe. For October 1 signifies the end of an era; it is the effective date of the final ICD-9-CM update before ICD-10-CM/PCS codes kick in on October 1, 2014. TOP MOVIES THE AVERAGE INCLUDED: COST OF A SUPERMAN THE NEW HOUSE MOVIE, THE DEER WAS $58,000 HUNTER, THE MUPPET MOVIE, ROCKY II “For those of us who have THE AVERAGE INCOME WAS MARGARET THATCHER WS been maintaining ICD-9-CM $17,500 ELECTED PRIM MINISTER IN since the code set’s THE UK implementation in 1979, this THE BOARD GAM TRIVIIAL THE SONY PURSUIT WAS LAUNCHED WALKMAN final ICD-9-CM code update is DEBUTED, VISICALC BECAME In 1979: RETAILING a historic occasion.” ICD-9-CM THE FIRST FOR $200 has one more year’s worth of SPREADSHEET PROGRAM POPULAR SONGS last calls, with coders using INCLUDED: “MY AVERAGE SHARONA” BY THE MONTHLY this latest and last code set KNACK; “HOT STUFF” AND A GALLON RENT WAS “BAD GIRLS BY GLORIA OF GAS $280 update until next October. A GAYNOR; PINK FLOYD WAS 86 RELEASED “THE WALL” CENTS reflective toast to ICD-9-CM. -

ADEX DENTAL EXAM SERIES: Fixed Prosthodontics and Endodontics
Developed by: Administered by: The American Board of The Commission on Dental Dental Examiners Competency Assessments ADEX DENTAL EXAM SERIES: Fixed Prosthodontics and Endodontics 2019 CANDIDATE MANUAL Please read all pertinent manuals in detail prior to attending the examination Copyright © 2018 American Board of Dental Examiners Copyright © 2018 The Commission on Dental Competency Assessments Ver 1.1- 2019 Exam Cycle Table of Contents Examination and Manual Overview 2 I. Examination Overview A. Manikin Exam Available Formats 4 B. Manikin Exam Parts 4 C. Endodontic and Prosthodontic Typodonts and Instruments 5 D. Examination Schedule Guidelines 6 1. Dates & Sites 6 2. Timely Arrival 6 E. General Manikin-Based Exam Administration Flow 7 1. Before the Exam: Candidate Orientation 7 2. Exam Day: Sample Schedule 7 3. Exam Day: Candidate Flow 8 F. Scoring Overview and Scoring Content 11 1. Section II. Endodontics Content 12 2. Section III. Fixed Prosthodontics Content 12 G. Penalties 13 II. Standards of Conduct and Infection Control A. Standards of Conduct 15 B. Infection Control Requirements 16 III. Examination Content and Criteria A. Endodontics Examination Procedures 19 B. Prosthodontics Examination Procedures 20 C. Endodontics Criteria 1. Anterior Endodontics Criteria 23 2. Posterior Endodontics Criteria 25 D. Prosthodontics Criteria 1. PFM Crown Preparation 27 2. Cast Metal Crown Preparation 29 3. Ceramic Crown Preparation 31 IV. Examination Forms A. Progress Form 34 See the Registration and DSE OSCE Manual for: • Candidate profile creation and registration • Online exam application process • DSE OSCE registration process and examination information / Prometric scheduling processes • ADEX Dental Examination Rules, Scoring, and Re-test processes 1 EXAMINATION AND MANUAL OVERVIEW The CDCA administers the ADEX dental licensure examination. -

Diagnostic Direct Laryngoscopy, Bronchoscopy & Esophagoscopy
Post-Operative Instruction Sheet Diagnostic Direct Laryngoscopy, Bronchoscopy & Esophagoscopy Direct Laryngoscopy: Examination of the voice box or larynx (pronounced “lair-inks”) under general anesthesia. An instrument called a laryngoscope is carefully placed into the mouth and used to visualize the larynx and surrounding structures. Bronchoscopy: Examination of the windpipe below the voice box in the neck and chest under general anesthesia. A long narrow telescope is passed through the larynx and used to carefully inspect the structures of the trachea and bronchi. Esophagoscopy: Examination of the swallowing pipe in the neck and chest under general anesthesia. An instrument called an esophagoscope is passed into the esophagus (just behind the larynx and trachea) and used to visualize the mucus membranes and surrounding structures of the esophagus. Frequently a small biopsy is taken to evaluate for signs of esophageal inflammation (esophagitis). What to Expect: Diagnostic airway endoscopy procedures generally take about 45 minutes to complete. Usually the procedure is well-tolerated and the child is back-to-normal the next day. Mild throat or tongue discomfort may persist for a few days after the procedure and is usually well-controlled with over-the-counter acetaminophen (Tylenol) or ibuprofen (Motrin). Warning Signs: Contact the office immediately at (603) 650-4399 if any of the following develop: • Worsening harsh, high-pitched noisy-breathing (stridor) • Labored breathing with chest retractions or flaring of the nostrils • Bluish discoloration of the lips or fingernails (cyanosis) • Persistent fever above 102°F that does not respond to Tylenol or Motrin • Excessive coughing or respiratory distress during feeding • Coughing or throwing up bright red blood • Excessive drowsiness or unresponsiveness Diet: Resume baseline diet (no special postoperative diet restrictions).