[Edit]Chemical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fleet Allocation - Period 4 2013-14
FLEET MOVEMENTS (PERIOD 3) RESERVE VEHICLES 16832 R832 OVN VOLVO OLYMPIAN Reserve to Disposal 21104 P604 JBU VOLVO B10BLE ST SPARE 16837 R837 OVN VOLVO OLYMPIAN Reserve to Disposal 21141 R241 KRG VOLVO B10BLE ST SPARE 16838 R838 OVN VOLVO OLYMPIAN Reserve to Disposal 21150 R250 KRG VOLVO B10BLE ST SPARE 16840 R840 OVN VOLVO OLYMPIAN Reserve to Disposal 22493 T493 BNL MAN ALX300 ST SPARE 33486 R466 SEF DENNIS DART SLF HA SPARE 91594 DX13 LUB VAUXHALL ASTRA New to Hartlepool 33491 R471 MVN DENNIS DART SLF HA SPARE Fleet DELICENCED VEHICLES Allocation for Hartlepool & Stockton VEHICLES FOR DISPOSAL 16832 R832 OVN VOLVO OLYMPIAN ST 16837 R837 OVN VOLVO OLYMPIAN ST 16838 R838 OVN VOLVO OLYMPIAN ST COMPANY VEHICLES 16840 R840 OVN VOLVO OLYMPIAN ST 91567* DU62 EKX VAUXHALL INSIGNIA OPs MANAGER ST 91594* DX13 LUB VAUXHALL ASTRA OPs MANAGER HA ANCILLARY VEHICLES KEY TO STORAGE DEPOTS HO = Head Office HA = Hartlepool ST = Stockton 93008* YE60 KMK FORD FIESTA CAR TRAFFIC HA 93013* YB60 HBD FORD FIESTA CAR ENGINEERING HA 93014* YB60 OAP FORD FIESTA CAR TRAFFIC ST 93025* YD61 WSO FORD FIESTA ENGINEERING ST 93300* YD61 UOJ FORD FIESTA TRAFFIC ST Address, Tel. & Fax No’s. 95174* MJ58 HVU FORD TRANSIT ENGINEERING ST 95190* YG10 KUP FORD TRANSIT ENGINEERING HA HEAD OFFICE * Vehicle leased from LEX Wheatsheaf, Sunderland SR5 1AQ Telephone: 0191 567 5251 Fax: 0191 566 0202 STOCKTON PERIOD 4 2013-14 Church Road, Stockton-on-Tees, TS18 2HW DRIVER TRAINING VEHICLES Telephone: 01642 602112 Fax: 01642 617733 20550 P550 ESA VOLVO B10M ST 20841 P841 GND VOLVO -
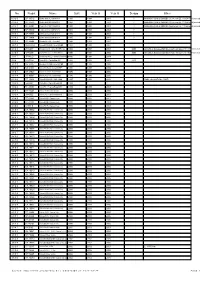
No. Regist Name Built Year O Year O Scrapp Other
No. Regist Name Built Year O Year O Scrapp Other 6325 SP 33512 Gräf & Stift GE110/54/57/A 1980 1998 2003 ? MAN/ÖAF/Gräf & Stift/BBC-Sécheron GE 110/54/57/A (according to Wikipedia) or MAN/Gräf & Stif GSGE 170 M18 (according to Norwegian bus list). 6326 SP 33530 Gräf & Stift GE110/54/57/A 1980 1998 2003 ? MAN/ÖAF/Gräf & Stift/BBC-Sécheron GE 110/54/57/A (according to Wikipedia) or MAN/Gräf & Stif GSGE 170 M18 (according to Norwegian bus list). 6327 SP 34082 Gräf & Stift GE110/54/57/A 1980 1998 2003 ? MAN/ÖAF/Gräf & Stift/BBC-Sécheron GE 110/54/57/A (according to Wikipedia) or MAN/Gräf & Stif GSGE 170 M18 (according to Norwegian bus list). 6333 SP 79839 Gräf & Stift SG200HO M18 1985 1998 2003 ? 6331 SP 79837 Gräf & Stift SG200HO M18 1985 1998 2003 ? 6332 SP 79838 Gräf & Stift SG200HO M18 1985 1998 2003 ? 6518 SP 94494 Scania N112CL / Arna M83 1986 1998 2001 2574 051-41 КА Volvo B10MA-55 / Arna M86BF 1987 1998 2001 2145 201 ART Scania K92CL / Arna M86BF-City 1988 1998 2003 2008 Arna Bruk Karosserifabrikken A/S, Arnatveit <b>3371</b> (1988) 2209 473 AZN Scania K113CLB / Arna M86BF 1990 1998 2006 2009 Arna Bruk Karosserifabrikken A/S, Arnatveit <b>3640</b> (1990) 1017 971 MJD Scania K113CLB / Carrus Classic II 3401992 1998 2004 500 678 TGO Volvo B12 / Carrus Star 501 1993 1998 2003 2013 2173 SR 52887 Scania L113CLB / Arna M91BF 1993 1998 2006 6189 SR 61994 Volvo B10B-60 / Arna M91BF 1994 1998 2006 6109 SR 67923 Volvo B10B-60 / Arna M91BF 1995 1998 2006 6114 SR 85567 Volvo B10B-60 / Arna M91BF 1996 1998 2006 6120 ST 11932 Volvo B10MA-55 / Säffle -

Fleet Archive
Fleet Archive 2020 15 March 2020 Repaints last week included Optare Solo M890/Optare 628 (NK61 DBZ) into “Little Coasters” livery. Volvo B9TL/Wright Eclipse Gemini 2 6004 (NK11 BHE) has also lost its branding for the “Red Arrows”, having been stripped of all vinyls, ahead of the introduction of new vehicles to this service in May. There were no fleet movements last week. 8 March 2020 Repaints last week included Mercedes Citaro 0350N/Mercedes Citaro 5278 (NK07 KPN) and 5279 (NK07 KPO) into the 2019 fleet livery. Scania N94UD/East Lancs OmniDekka 6143 (YN04 GKA) is no longer a float/reserve vehicle and now forms part of the main fleet allocation at Riverside. It has replaced former East Yorkshire Volvo B7TL/Plaxton President 6935 (X508 EGK) which has suffered defects uneconomical to repair. Float Optare Solo M890/Optare 636 (NK61 FMD) is now allocated to Percy Main to provide cover for the remaining “Little Coasters” branded Optare Solo repaints. Scania L94UB/Wright Solar 5226 (NK54 NVZ) has now been withdrawn from service at Riverside and, together with 5231 (NK55 OLJ), has transferred to East Yorkshire on temporary loan. 1 March 2020 The final coach to be repainted as part of the ongoing work into the new Northern Coaching unit is Scania K340EB/Caetano Levante 7098 (JCN 822) into Voyager livery. Notable is the allocation of the registration mark JCN822: this registration mark being allocated to Leyland Tiger/Plaxton Paramount 7038 (E116 KFV) from 1990 to 1997 whilst a part of the Northern fleet in Voyager livery. Scania N94UD/East Lancs OmniDekka 6143 (YN04 GKA) has transferred from Chester-le-Street to Riverside, as a float/reserve vehicle. -

Arriva Yorkshire Fleet List
© http://westyorkshirebusphotos.wordpress.com/ 31/05/2018 Arriva Yorkshire Fleet Registration Body Chassis Seat Livery Depot Notes Num. Code 261 SN55 HTY Plaxton Pointer 2 Dennis Dart SLF B29F Arriva Interurban Selby 262 SN55 HTZ Plaxton Pointer 2 Dennis Dart SLF B29F Arriva Interurban Selby 401 K401 HWW Alexander Strider Volvo B10B N/A Driver Trainer Wakefield Driver Training 403 K403 HWW Alexander Strider Volvo B10B N/A Driver Trainer Wakefield Driver Training 499 YJ04 HJG Wright Commander DAF SB200 B44F Driver Trainer Wakefield Driver Training 703 YD02 PXZ Optare Spectra DAF DB250 H47/27F Arriva Interurban Wakefield 704 YG52 CFA Optare Spectra DAF DB250 H47/27F Driver Trainer Wakefield Driver Training 723 YD02 PYZ Optare Spectra DAF DB250 H47/27F Arriva Interurban Wakefield 1001 YY14 LFM Alexander Enviro200 ADL Enviro 200 B28F Arriva MAX (Big In Yorkshire) Castleford 1002 YY14 LFN Alexander Enviro200 ADL Enviro 200 B28F Arriva MAX (Big In Yorkshire) Castleford 1003 YY14 LFO Alexander Enviro200 ADL Enviro 200 B28F Arriva MAX (Big In Yorkshire) Castleford 1004 YY14 LFP Alexander Enviro200 ADL Enviro 200 B28F Arriva MAX (Big In Yorkshire) Castleford 1005 YY14 LFR Alexander Enviro200 ADL Enviro 200 B28F Arriva MAX (Big In Yorkshire) Wakefield 1006 YY14 LFS Alexander Enviro200 ADL Enviro 200 B28F Arriva MAX (Big In Yorkshire) Wakefield 1007 YY14 LFT Alexander Enviro200 ADL Enviro 200 B28F Arriva MAX (Big In Yorkshire) Wakefield 1008 YY14 LFU Alexander Enviro200 ADL Enviro 200 B28F Arriva MAX (Big In Yorkshire) Wakefield 1009 YY14 LFV Alexander -

Fleet Allocation
FLEET MOVEMENTS (PERIOD 13) DRIVER TRAINING VEHICLES 20551 P551 ESA VOLVO B10M S/Shields to Driver Trainer 20551 P551 ESA VOLVO B10M WA CONVERSION TO D/T 20553 P553 ESA VOLVO B10M S/Shields to Driver Trainer 20553 P553 ESA VOLVO B10M WA CONVERSION TO D/T 21101 P601 JBU VOLVO B10BLE S/Shields to Walkergate 20554 P554 ESA VOLVO B10M WA 21032/4/6 L32-36 HHN VOLVO B10B WA Awaiting Scrap The following VOLVO B10BLE’s have gone from S/Shields to Reserve: 21041/2 M41/2 PVN VOLVO B10B SS/SU Awaiting Scrap 21138 R238 KRG 21148 R248 KRG 21154 R254 KRG 32125 K125 SRH DENNIS DART SL Awaiting Disposal 21145 R245 KRG 21150 R250 KRG 21156 R256 KRG 21146 R246 KRG 21153 R253 KRG 21157 R257 KRG RESERVE VEHICLES The following ADL ENVIRO 300’s are new to S/Shields Volvo Olympians Volvo B10BLE cont 27723 NK11 BFJ 27729 NK11 BFU 27734 NK11 BGE 16704 N704 LTN WA Spare 21155 R255 KRG WA Spare 27724 NK11 BFL 27730 NK11 BFV 27735 NK11 BGF 16705 N705 LTN WA Spare 21156 R256 KRG SS Spare 27725 NK11 BFM 27731 NK11 BFX 27736 NK11 BGO 16707 N707 LTN WA Spare 21157 R257 KRG SS Spare 27726 NK11 BFN 27732 NK11 BFY 27737 NK11 BGU 16730 N730 LTN WA Spare Dennis Dart SLF 27727 NK11 BFO 27733 NK11 BFZ 27738 NK11 BGV Volvo B10M (*B10B) 33101 R101 KRG SS Spare 27728 NK11 BFP 20126 P126 XCN SL Trans. 33118 R118 KRG SU Chassis Fleet 32125 K125 SRH DENNIS DART D/Trainer to Disposal 20253 R653 RPY SL Spare 33489 R469 MVN SS Spare 32624 P624 PGP DENNIS DART Sold for Scrap 20258 R558 RPY SL Spare 33492 R472 MVN SS Spare 33118 K118 KRG DENNIS DART SLF Sunderland to Reserve 20263 N553 VDC SL Spare 33825 R825 YUD SS Spare 33489 R469 MVN DENNIS DART SLF S/Shields to Reserve 20264 R554 RPY SL Spare 33828 R828 YUD SS Spare 33492 R472 MVN DENNIS DART SLF S/Shields to Reserve 20840 P840 GND SL Engine 33829 R829 YUD SU Chassis 33825 R825 YUD DENNIS DART SLF S/Shields to Reserve 20842 P842 GND WA Engine Designline H.E.V. -

26 February 2010
5 March 2018 Ex-London Volvo B9TL/Wright Gemini 6160: LJ62 KZD has been delivered while 6161: LJ62 KZP has entered service at Stanley. Optare Versa 8299: NK09 FUY is now in service at Chester-le-Street while Scania L94s 4957: NL52 WVN & 4959: NL52 WVP and Volvo B7TL/Plaxton 6017: V317 LGC have been withdrawn. Volvo B7TL/Plaxton 6032: V332 LGC has been sold to Alpha Recovery, Weetslade. 26 February 2018 Two more ex-London Volvo B9TL/Wright to be received are 6149: LJ62 KBY and 6157: LJ62 KXZ. Special events vehicles 3973: PJ02 PYZ and 3983: PJ02 PZL have been transferred to the main Riverside fleet; Scania L94/Wright 4945: NK51 OLJ has been returned to service at Hexham and Optare Versa 8296: NK09 FUU has been transferred from Riverside to Chester le Street. Volvo B7TL/East Lancs 3980: PJ02 PZG and Scania L94/Wright 4943: NK51 OLG have been sold to Alpha Recovery, Weetslade. 19 February 2018 The first of 13 Volvo B9TL with Wright Gemini bodies to be acquired from Go-ahead London is 6161: LJ62 KZP which is in fleet livery and is being prepared for service at Stanley. Percy Main has transferred Scania Omnicity 5252: NK56 KHY to Stanley who have transferred Scania Omndekka 6135: YN04 GJV to Percy Main. Optare Versa 8297: NK09 FUV is now in service at Chester le Street and Scania L94/Wright 4945: NK51 OLJ has been withdrawn. Also withdrawn are Scania L94/Wright 4974: NK53 UOA and Volvo B7TL/Plaxton 6015: V315 LGC from Percy Main and Scania Omnidekka 6130: GX03 SVE from Stanley. -
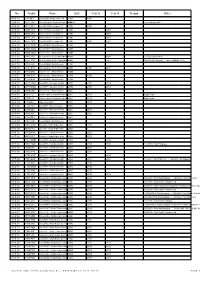
No. Regist Name Built Year O Year O Scrapp Other
No. Regist Name Built Year O Year O Scrapp Other 40512 L971NET Dennis Dart / Plaxton Pointer 9.8 1993 1997 30552 N312 JBV Volvo Olympian / Northern Counties Palatine1995 II ex-Centrewest V12 60556 M719 VET Volvo B10M-55 / Alexander PS 1995 1998 60596 N759 CKY Volvo B10M-55 / Alexander PS 1996 ? 2007 60579 N742 CKY Volvo B10M-55 / Alexander PS 1996 ? 2007 60590 N753 CKY Volvo B10M-55 / Alexander PS 1996 ? 2007 60594 N757 CKY Volvo B10M-55 / Alexander PS 1996 1998 2007 60618 R781 WKW Volvo B10BLE / Wright Renown 1997 60620 R783 WKW Volvo B10BLE / Wright Renown 1997 60622 R785 WKW Volvo B10BLE / Wright Renown 1997 52592 R412 VPU Mercedes-Benz 814 D / Marshall Master1998 ? 2008 ex Essex Buses 412 50192 R125 XDT Mercedes-Benz 814 D / Plaxton Beaver1998 2 ? 2007 First Sheffield depot <br>ex Mainline 125 66114 R914 BOU Volvo B10BLE / Wright Renown 1998 50205 KTA 16112 Mercedes-Benz 814 D / Plaxton Beaver1998 2 1998 2011 60660 T842 MAK Volvo B10BLE / Wright Renown 1999 60687 T869 ODT Volvo B10BLE / Wright Renown 1999 1999 60650 T832 MAK Volvo B10BLE / Wright Renown 1999 30877 W732 DWX Volvo B7TL / Alexander ALX400 2000 2002 2013 30876 W731 DWX Volvo B7TL / Alexander ALX400 2000 2000 2013 62240 Y949 CSF Volvo B10BLE / Wright Renown 2001 32955 X611 HLT Dennis Trident 2 / Plaxton President 2001 2011 2016 Dagenham 32957 X957 HLT Dennis Trident 2 / Plaxton President 2001 2011 Dagenham 50234 Y253HHL Optare Solo M850 2001 2001 32108 LT02 ZCY Volvo B7TL / Plaxton President 2001 30562 X857UOK Volvo B7TL / Alexander ALX400 2001 2001 30563 X858UOK Volvo -

Fleet Archive
Fleet Archive 2019 22 April 2019 There were no full repaints last week, but Optare Solo SR M925/Optare 673 (NK16 BXP) has been fitted with USB charging points at Thornton Bros, Ashington, and has now been returned to Go North East for repaint. “Hadrian’s Wall Country Bus” branded Optare Solo SR M890/Optare 635 (NK61 FJP) and 636 (NK61 FXD) have both returned to service at Hexham for this year’s operation of the seasonal AD122 service. 15 April 2019 Buses repainted last week included Optare Solo SR M925/Optare 672 (NK16 BXO) into the 2019 fleet livery. It was also fitted with USB charging points at Thornton Bros, Ashington. Scania K340EB/Caetano Levante 7094 (FJ08 KLF) has been sold to Ensign Bus Co., Purfleet. 8 April 2019 Buses repainted last week included Optare Solo SR M925/Optare 690 (NK66 CXF) into pink (subsequently receiving "indiGo Washington" branding). After two and a half years of development, the region’s largest bus operator, Go North East opened the doors to its brand new £3.5 million, state-of-the-art Hownsgill Depot in Consett, County Durham, on Friday 15 March 2019. The high-tech operation replaces the company’s Stanley Depot, which it operated from for nearly 100 years. Therefore; most vehicles based at Stanley transferred to Hownsgill on Sunday 7 April, the date from which the new depot commenced operations. In exchange for 5234 – 5237, ex-Go South Coast Mercedes Citaros 5482 – 5485 have transferred from Saltmeadows to Hownsgill. Likewise, Mercedes Citaro 5481 has transferred from Riverside to Hownsgill in exchange for Scania OmniCity 5238. -

First South West - Buses of Somerset, First Kernow (PH0004983) First South West Limited, Union Street, Camborne, Cornwall, TR14 8HF
First South West - Buses of Somerset, First Kernow (PH0004983) First South West Limited, Union Street, Camborne, Cornwall, TR14 8HF Part of First Group PLC. Depots: Buses of Somerset Bridgwater Boards Road, Bridgwater, Somerset, TA6 4BB Taunton Hamilton Road, Taunton, Somerset, TA1 2EH Yeovil 23 Reckleford, Yeovil, Somerset, BA21 4EJ First Kernow Camborne Union Street, Camborne, Cornwall, TR14 8HF Newquay Western House, St Austell Street, Summercourt, Newquay, Cornwall, TR8 5DR Penzance Long Rock Industrial Estate, Penzance, Cornwall, TR20 8HZ Truro Unit 4, Lighteridge Hill, Newham, Truro, Cornwall, TR1 2XR Outstations: Buses of Somerset Minehead Venners Yard, Brunnel Way, Minehead, Somerset, TA24 5BJ First Kernow Bodmin Springpark Workshops, Old Callywith Road, Bodmin, Cornwall, PL31 2DZ Callington Duchy College Stoke Climsland, Stoke Climsland, Callington, Devon, PL17 8PD FRBP Limitefd, Block A, Florence Road, Business Park, Kelly Bray, Callington, Devon, PL17 8EX Exeter Hill Barton Business Park, Sidmouth Road, Clyst St Mary, Exeter, Devon, EX5 1DR Falmouth Tregoniggie Industrial Estate, Falmouth, Cornwall, TR11 4SN Helston The Flambards Experience, Helston, Cornwall, TR13 0QA Newquay Tresillian Barton, Summercourt, Newquay, Cornwall, TR8 5AA Padstow Field Next Door to Tesco, Trevethan Farm, Sarah’s Lane, Padstow, Cornwall, TL28 8LE Plymouth Lee Moor Workshops, Lee Moor, Plymouth, Devon, PL7 5JA Plymouth Railway Station, North Road, Plymouth, Devon, PL4 6AB The Eden Project Bodelva, Par, Cornwall, PL24 2SG Winkleigh The Airfield, Winkleigh, -

Magasinet Bus Om Deres Ar- Bejde, Der Blandt Andet Går Ud På at Levere En God Service
MMaaggaassiinneett BBuuss Det er et ønskejob at være buschauffør Læs mere side 12 - 15 MAN leverer gasbusser til Sverige og Danmark Læs mere side 20 - 21 Passagerer, chauffører og beboere er tilfredse med hybridbus Læs mere side 18 - 19 Danish Coach Award-vinder: Letbane i Frankrig Klingenberg Rejser Byen bliver helt forandret er specialister Trafikken blev lagt om i busrejser Driftselskab har en kontrakt på 30 år Læs side 32-33 Læs side 26 - 31 Hvor godtfolk er, kommer godtfolk til En kundetilfredshedsundersøgelse fra Fyn viser stigende tilfredshed med FynBus. Også med FynBus’ chauffører. To buschauffører fra Midtjylland fortæller i dette nummer af Magasinet Bus om deres ar- bejde, der blandt andet går ud på at levere en god service. Både kundetilfredshedsundersøgelsen og de to chaufførers udtalelser peger i samme retning - at chaufførerne har en afgørende indflydelse på, hvordan passagererne opfatter bustransport. Chaufførerne er også i centrum hos busoperatører i Hovedstaden og andre steder. Og det gælder ikke blot chaufførernes direkte service over for passagererne. det gælder også den måde, de kører bussen på. Det gør rejsen behagelig for passagerer - og den økonomiske rejse for busoperatørerne bliver også bedre. Endnu en gang et eksempel på, at tingene hænger sammen. Men kan busoperatører blive ved med at finde chauffører, der kan leve op til forretnin- gerne? Det er nok en af de væsentligste udfordringer i de kommende år, hvor gennemsnitsal- deren blandt chauffører er stigende. Hvad kan man gøre for at tiltrække nye chauffører? Bedre løn? lønnen spiller ind, men gør det ikke alene Bedre arbejdsmiljø? ja, men det gør det ikke alene Større respekt fra andre over for jobbet som chauffør? ja, men det gør det ikke alene Listen kan sikkert gøres længere, men de tre punkter sammen er ganske afgørende - særligt det sidste. -
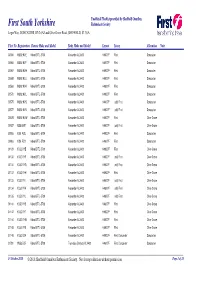
Fleet List \251 Sheffield Omnibus Enthusiasts Society
Unofficial Fleetlist provided by Sheffield Omnibus First South Yorkshire Enthusiasts Society Leger Way, DONCASTER, DN2 6AZ and Olive Grove Road, SHEFFIELD, S2 3GA Fleet No Registration Chassis Make and Model Body Make and Model Layout Livery Allocation Note 30564 WU02 KVE Volvo B7TL-5700 Alexander ALX400 H49/27F First Doncaster 30565 WU02 KVF Volvo B7TL-5700 Alexander ALX400 H49/27F First Doncaster 30567 WU02 KVH Volvo B7TL-5700 Alexander ALX400 H49/27F First Doncaster 30568 WU02 KVJ Volvo B7TL-5700 Alexander ALX400 H49/27F First Doncaster 30569 WU02 KVK Volvo B7TL-5700 Alexander ALX400 H49/27F First Doncaster 30570 WU02 KVL Volvo B7TL-5700 Alexander ALX400 H49/27F First Doncaster 30575 WU02 KVS Volvo B7TL-5700 Alexander ALX400 H49/27F (old) First Doncaster 30577 WU02 KVV Volvo B7TL-5700 Alexander ALX400 H49/27F (old) First Doncaster 30578 WU02 KVW Volvo B7TL-5700 Alexander ALX400 H49/27F First Olive Grove 30937 X358 VWT Volvo B7TL-5700 Alexander ALX400 H49/27F (old) First Olive Grove 30955 YJ51 RCU Volvo B7TL-5700 Alexander ALX400 H49/27F First Doncaster 30963 YJ51 RDY Volvo B7TL-5700 Alexander ALX400 H49/27F First Doncaster 31129 YU52 VYE Volvo B7TL-5700 Alexander ALX400 H49/27F First Olive Grove 31130 YU52 VYF Volvo B7TL-5700 Alexander ALX400 H49/27F (old) First Olive Grove 31131 YU52 VYG Volvo B7TL-5700 Alexander ALX400 H49/27F (old) First Olive Grove 31132 YU52 VYH Volvo B7TL-5700 Alexander ALX400 H49/27F First Olive Grove 31133 YU52 VYJ Volvo B7TL-5700 Alexander ALX400 H49/27F (old) First Olive Grove 31134 YU52 VYK Volvo B7TL-5700 -

Magasinet-Bus-3-2014 Kopi
Magasinet Bus Onsdag 26. marts 2014 - Nummer 3 - 2. årgang Specialkørsel kræver specielle talenter Læs mere side 15 - 18 Den kollektive trafik Bilingeniør: kører frem Det ville være mindre farligt at køre Læs mere side 9 uden sele Konklusion: Prisværdigt Læs mere side 26 Konkurrencen mellem Papuga i Brørup har busoperatører en rullende innovation fungerer Læs mere side 11 Læs mere side 34 - 40 Busser kørte ind i landsdækkende kontrol Læs mere side 22 Mød os på Facebook Følg med hver dag på transportnyhederne.dk 2! Sikkerhed eller falsk tryghed I dette nummer skriver vi endnu en gang om sikkerhedsseler i børnehavebusser. Trafikstyrelsen har bedt synsvirksomheder over hele landet om at sikre, at 115 busser, der er indrettet til at køre med børn. Det handler i vid udstrækning om tidligere bybusser eller rutebusser, som har fået sat nogle sikkerhedsseler fast på de eksisterende sæder, fået sat nogle borde ind mellem sæderne og måske et køkken i bagenden. Vi har set nogle af busserne, som er ved at blive bygget om - eller skal bygges om. Busserne har kørt med børn - de har haft seler monteret. Men har de givet den sikkerhed, som man kan forvente - eller har de blot givet falsk tryghed. Når det gælder børnehavebusserne, er der udsigt til en forbedret sikkerhed. Men hvad med de busser, som private vognmænd har fået sat sikkerhedsseler i - og så dagligt bruges til at køre børn til og fra skoler, klubber, SFO’ere og idrætsaktiviteter? Det kan være flere hundrede, som Trafikstyrelsens fokus ikke er rettet ind på i første omgang. God læselyst i Magasinet Bus - 3 - 2014.