Systematic Review Adherence to Methodological Or Reporting Quality
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Health Economics & Decision Science
School of Health And Related Research Health Economics & Decision Science (HEDS) Discussion Paper Series Factors influencing the use of research for policy-making: an umbrella review protocol Authors: Erika E. Atienzo, Eva Kaltenthaler, Susan K. Baxter Corresponding author: Erika E. Atienzo ScHARR, University of Sheffield, Regent Court, 30 Regent Street, Sheffield, S1 4DA Fax: (+44) (0)114 222 0749 Email: [email protected] No. 16.07 Disclaimer: This series is intended to promote discussion and to provide information about work in progress. The views expressed in this series are those of the authors. Comments are welcome, and should be sent to the corresponding author. This paper is also hosted on the White Rose Repository: http://eprints.whiterose.ac.uk/ FACTORS INFLUENCING THE USE OF RESEARCH FOR POLICY-MAKING: AN UMBRELLA REVIEW PROTOCOL Erika E. Atienzoa*, Eva Kaltenthalera, Susan K. Baxterb a Health Economics and Decision Science, School of Health and Related Research, University of Sheffield b Public Health, School of Health and Related Research, University of Sheffield *Corresponding author: Erika E. Atienzo ScHARR, University of Sheffield Regent Court, 30 Regent Street Sheffield, S1 4DA (UK) [email protected] March 2016 ABSTRACT Over the last two decades, there has been an emphasis on the concept of evidence-based policy. However, evidence-based policy remains a major challenge and a gap exists in the systematic translation of scientific knowledge into policies. The awareness of this evidence- policy gap has led to a proliferation of research. As the demand for evidence-informed policy-making escalates, so does the need to unveil the mechanisms by which we can influence the process of research uptake. -

Those Missing Values in Questionnaires
Those Missing Values in Questionnaires John R. Gerlach, Maxim Group, Plymouth Meeting, PA Cindy Garra, IMS HEALTH; Plymouth Meeting, PA Abstract alternative values are not bona fide SAS missing values; consequently, a SAS procedure, expectedly, will include Questionnaires are notorious for containing these alternative values, thereby producing bogus results. responses that later become missing values during processing and analyses. Contrary to a non-response that Typically, you can re-code the original data, so results in a typical, bona fide, missing value, questions that the missing values become ordinary missing values, might allow several alternative responses, necessarily thus allowing SAS to process only appropriate values. Of outside the usual range of appropriate responses. course, there's a loss of information since the original Whether a question represents continuous or categorical missing values did not constitute a non-response. Also, data, a good questionnaire offers meaningful alternatives, such pre-processing might include a myriad of IF THEN / such as: "Refused to Answer" and, of course, the ELSE statements, which can be very tedious and time- quintessential "Don't Know." Traditionally, these consuming to write, and difficult to maintain. Thus, given alternative responses have numeric values such as 97, a questionnaire that has over a hundred variables with 998, or 9999 and, therefore, pose problems when trying varying levels of missing values, the task of re-coding to distinguish them from normal responses, especially these variables becomes very time consuming at best. when multiple missing values exist. This paper discusses Even worse, the permanent re-coding of alternative missing values in SAS and techniques that facilitate the responses to ordinary missing numeric values in SAS process of dealing with multi-valued, meaningful missing precludes categorical analysis that requires the inclusion values often found in questionnaires. -

10 Questions Opinion Polls
Questions you might have on 10opinion polls 1. What is an opinion poll? An opinion poll is a survey carried out to measure views on a certain topic within a specific group of people. For example, the topic may relate to who Kenyans support in the presidential race, in which case, the group of people interviewed will be registered voters. 2. How are interviewees for an opinion poll selected? The group of people interviewed for an opinion poll is called a sample. As the name suggests, a sample is a group of people that represents the total population whose opinion is being surveyed. In a scientific opinion poll, everyone has an equal chance of being interviewed. 3. So how come I have never been interviewed for an opinion poll? You have the same chance of being polled as anyone else living in Kenya. However, chances of this are very small and are estimated at about 1 in 14,000. This is because there are approximately 14 million registered voters in Kenya and, for practical and cost reasons, usually only between 1,000 and 2,000 people are interviewed for each survey carried out. 4. How can such a small group be representative of the entire population? In order to ensure that the sample/survey group is representative of the population, the surveyors must ensure that the group reflects the characteristics of the whole. For instance, to get a general idea of who might win the Kenyan presidential election, only the views of registered voters in Kenya will be surveyed as these are the people who will be able to influence the election. -

Handbook of Recommended Practices for Questionnaire Development and Testing in the European Statistical System
Handbook of Recommended Practices for Questionnaire Development and Testing in the European Statistical System Release year: 2006 Authors: G. Brancato, S. Macchia, M. Murgia, M. Signore, G. Simeoni - Italian National Institute of Statistics, ISTAT K. Blanke, T. Körner, A. Nimmergut - Federal Statistical Office Germany, FSO P. Lima, R. Paulino - National Statistical Institute of Portugal, INE J.H.P. Hoffmeyer-Zlotnik - German Center for Survey Research and Methodology, ZUMA Version 1 Acknowledgements We are grateful to the experts from the network countries who supported us in all relevant stages of the work: Anja Ahola, Dirkjan Beukenhorst, Trine Dale, Gustav Haraldsen. We also thank all colleagues from European and overseas NSIs who helped us in understanding the current practices and in the review of the draft version of the handbook. Executive summary Executive Summary Questionnaires constitute the basis of every survey-based statistical measurement. They are by far the most important measurement instruments statisticians use to grasp the phenomena to be measured. Errors due to an insufficient questionnaire can hardly be compensated at later stages of the data collection process. Therefore, having systematic questionnaire design and testing procedures in place is vital for data quality, particularly for a minimisation of the measurement error. Against this background, the Directors General of the members of the European Statistical System (ESS) stressed the importance of questionnaire design and testing in the European Statistics Code of Practice, endorsed in February 2005. Principle 8 of the Code states that “appropriate statistical procedures, implemented from data collection to data validation, must underpin quality statistics.” One of the indicators referring to this principle requires that “questionnaires are systematically tested prior to the data collection.” Taking the Code of Practice as a starting point, this Recommended Practice Manual aims at further specifying the requirements of the Code of Practice. -

Meta-Analysis of the Relationships Between Different Leadership Practices and Organizational, Teaming, Leader, and Employee Outcomes*
Journal of International Education and Leadership Volume 8 Issue 2 Fall 2018 http://www.jielusa.org/ ISSN: 2161-7252 Meta-Analysis of the Relationships Between Different Leadership Practices and Organizational, Teaming, Leader, and Employee Outcomes* Carl J. Dunst Orelena Hawks Puckett Institute Mary Beth Bruder University of Connecticut Health Center Deborah W. Hamby, Robin Howse, and Helen Wilkie Orelena Hawks Puckett Institute * This study was supported, in part, by funding from the U.S. Department of Education, Office of Special Education Programs (No. 325B120004) for the Early Childhood Personnel Center, University of Connecticut Health Center. The contents and opinions expressed, however, are those of the authors and do not necessarily reflect the policy or official position of either the Department or Office and no endorsement should be inferred or implied. The authors report no conflicts of interest. The meta-analysis described in this paper evaluated the relationships between 11 types of leadership practices and 7 organizational, teaming, leader, and employee outcomes. A main focus of analysis was whether the leadership practices were differentially related to the study outcomes. Studies were eligible for inclusion if the correlations between leadership subscale measures (rather than global measures of leadership) and outcomes of interest were reported. The random effects weighted average correlations between the independent and dependent measures were used as the sizes of effects for evaluating the influences of the leadership practices on the outcome measures. One hundred and twelve studies met the inclusion criteria and included 39,433 participants. The studies were conducted in 31 countries in different kinds of programs, organizations, companies, and businesses. -
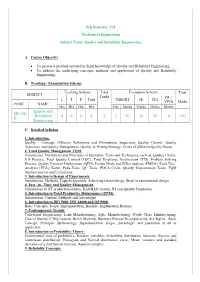
D Quality and Reliability Engineering 4 0 0 4 4 3 70 30 20 0
B.E Semester: VII Mechanical Engineering Subject Name: Quality and Reliability Engineering A. Course Objective To present a problem oriented in depth knowledge of Quality and Reliability Engineering. To address the underlying concepts, methods and application of Quality and Reliability Engineering. B. Teaching / Examination Scheme Teaching Scheme Total Evaluation Scheme Total SUBJECT Credit PR. / L T P Total THEORY IE CIA VIVO Marks CODE NAME Hrs Hrs Hrs Hrs Hrs Marks Marks Marks Marks Quality and ME706- Reliability 4 0 0 4 4 3 70 30 20 0 120 D Engineering C. Detailed Syllabus 1. Introduction: Quality – Concept, Different Definitions and Dimensions, Inspection, Quality Control, Quality Assurance and Quality Management, Quality as Wining Strategy, Views of different Quality Gurus. 2. Total Quality Management TQM: Introduction, Definitions and Principles of Operation, Tools and Techniques, such as, Quality Circles, 5 S Practice, Total Quality Control (TQC), Total Employee Involvement (TEI), Problem Solving Process, Quality Function Deployment (QFD), Failure Mode and Effect analysis (FMEA), Fault Tree Analysis (FTA), Kizen, Poka-Yoke, QC Tools, PDCA Cycle, Quality Improvement Tools, TQM Implementation and Limitations. 3. Introduction to Design of Experiments: Introduction, Methods, Taguchi approach, Achieving robust design, Steps in experimental design 4. Just –in –Time and Quality Management: Introduction to JIT production system, KANBAN system, JIT and Quality Production. 5. Introduction to Total Productive Maintenance (TPM): Introduction, Content, Methods and Advantages 6. Introduction to ISO 9000, ISO 14000 and QS 9000: Basic Concepts, Scope, Implementation, Benefits, Implantation Barriers 7. Contemporary Trends: Concurrent Engineering, Lean Manufacturing, Agile Manufacturing, World Class Manufacturing, Cost of Quality (COQ) system, Bench Marking, Business Process Re-engineering, Six Sigma - Basic Concept, Principle, Methodology, Implementation, Scope, Advantages and Limitation of all as applicable. -

Survey Experiments
IU Workshop in Methods – 2019 Survey Experiments Testing Causality in Diverse Samples Trenton D. Mize Department of Sociology & Advanced Methodologies (AMAP) Purdue University Survey Experiments Page 1 Survey Experiments Page 2 Contents INTRODUCTION ............................................................................................................................................................................ 8 Overview .............................................................................................................................................................................. 8 What is a survey experiment? .................................................................................................................................... 9 What is an experiment?.............................................................................................................................................. 10 Independent and dependent variables ................................................................................................................. 11 Experimental Conditions ............................................................................................................................................. 12 WHY CONDUCT A SURVEY EXPERIMENT? ........................................................................................................................... 13 Internal, external, and construct validity .......................................................................................................... -

Quality Assurance: Best Practices in Clinical SAS® Programming
NESUG 2012 Management, Administration and Support Quality Assurance: Best Practices in Clinical SAS® Programming Parag Shiralkar eClinical Solutions, a Division of Eliassen Group Abstract SAS® programmers working on clinical reporting projects are often under constant pressure of meeting tight timelines, producing best quality SAS® code and of meeting needs of customers. As per regulatory guidelines, a typical clinical report or dataset generation using SAS® software is considered as software development. Moreover, since statistical reporting and clinical programming is a part of clinical trial processes, such processes are required to follow strict quality assurance guidelines. While SAS® programmers completely focus on getting best quality deliverable out in a timely manner, quality assurance needs often get lesser priorities or may get unclearly understood by clinical SAS® programming staff. Most of the quality assurance practices are often focused on ‘process adherence’. Statistical programmers using SAS® software and working on clinical reporting tasks need to maintain adequate documentation for the processes they follow. Quality control strategy should be planned prevalently before starting any programming work. Adherence to standard operating procedures, maintenance of necessary audit trails, and necessary and sufficient documentation are key aspects of quality. This paper elaborates on best quality assurance practices which statistical programmers working in pharmaceutical industry are recommended to follow. These quality practices -

MRS Guidance on How to Read Opinion Polls
What are opinion polls? MRS guidance on how to read opinion polls June 2016 1 June 2016 www.mrs.org.uk MRS Guidance Note: How to read opinion polls MRS has produced this Guidance Note to help individuals evaluate, understand and interpret Opinion Polls. This guidance is primarily for non-researchers who commission and/or use opinion polls. Researchers can use this guidance to support their understanding of the reporting rules contained within the MRS Code of Conduct. Opinion Polls – The Essential Points What is an Opinion Poll? An opinion poll is a survey of public opinion obtained by questioning a representative sample of individuals selected from a clearly defined target audience or population. For example, it may be a survey of c. 1,000 UK adults aged 16 years and over. When conducted appropriately, opinion polls can add value to the national debate on topics of interest, including voting intentions. Typically, individuals or organisations commission a research organisation to undertake an opinion poll. The results to an opinion poll are either carried out for private use or for publication. What is sampling? Opinion polls are carried out among a sub-set of a given target audience or population and this sub-set is called a sample. Whilst the number included in a sample may differ, opinion poll samples are typically between c. 1,000 and 2,000 participants. When a sample is selected from a given target audience or population, the possibility of a sampling error is introduced. This is because the demographic profile of the sub-sample selected may not be identical to the profile of the target audience / population. -

Data Quality Monitoring and Surveillance System Evaluation
TECHNICAL DOCUMENT Data quality monitoring and surveillance system evaluation A handbook of methods and applications www.ecdc.europa.eu ECDC TECHNICAL DOCUMENT Data quality monitoring and surveillance system evaluation A handbook of methods and applications This publication of the European Centre for Disease Prevention and Control (ECDC) was coordinated by Isabelle Devaux (senior expert, Epidemiological Methods, ECDC). Contributing authors John Brazil (Health Protection Surveillance Centre, Ireland; Section 2.4), Bruno Ciancio (ECDC; Chapter 1, Section 2.1), Isabelle Devaux (ECDC; Chapter 1, Sections 3.1 and 3.2), James Freed (Public Health England, United Kingdom; Sections 2.1 and 3.2), Magid Herida (Institut for Public Health Surveillance, France; Section 3.8 ), Jana Kerlik (Public Health Authority of the Slovak Republic; Section 2.1), Scott McNabb (Emory University, United States of America; Sections 2.1 and 3.8), Kassiani Mellou (Hellenic Centre for Disease Control and Prevention, Greece; Sections 2.2, 2.3, 3.3, 3.4 and 3.5), Gerardo Priotto (World Health Organization; Section 3.6), Simone van der Plas (National Institute of Public Health and the Environment, the Netherlands; Chapter 4), Bart van der Zanden (Public Health Agency of Sweden; Chapter 4), Edward Valesco (Robert Koch Institute, Germany; Sections 3.1 and 3.2). Project working group members: Maria Avdicova (Public Health Authority of the Slovak Republic), Sandro Bonfigli (National Institute of Health, Italy), Mike Catchpole (Public Health England, United Kingdom), Agnes -

A Meta-Review of Transparency and Reproducibility-Related Reporting Practices in Published Meta- Analyses on Clinical Psychological Interventions (2000-2020)
A meta-review of transparency and reproducibility-related reporting practices in published meta- analyses on clinical psychological interventions (2000-2020). Rubén López-Nicolás1, José Antonio López-López1, María Rubio-Aparicio2 & Julio Sánchez- Meca1 1 Universidad de Murcia (Spain) 2 Universidad de Alicante (Spain) Author note: This paper has been published at Behavior Research Methods. The published version is available at: https://doi.org/10.3758/s13428-021-01644-z. All materials, data, and analysis script coded have been made publicly available on the Open Science Framework: https://osf.io/xg97b/. Correspondence should be addressed to Rubén López-Nicolás, Facultad de Psicología, Campus de Espinardo, Universidad de Murcia, edificio nº 31, 30100 Murcia, España. E-mail: [email protected] 1 Abstract Meta-analysis is a powerful and important tool to synthesize the literature about a research topic. Like other kinds of research, meta-analyses must be reproducible to be compliant with the principles of the scientific method. Furthermore, reproducible meta-analyses can be easily updated with new data and reanalysed applying new and more refined analysis techniques. We attempted to empirically assess the prevalence of transparency and reproducibility-related reporting practices in published meta-analyses from clinical psychology by examining a random sample of 100 meta-analyses. Our purpose was to identify the key points that could be improved with the aim to provide some recommendations to carry out reproducible meta-analyses. We conducted a meta-review of meta-analyses of psychological interventions published between 2000 and 2020. We searched PubMed, PsycInfo and Web of Science databases. A structured coding form to assess transparency indicators was created based on previous studies and existing meta-analysis guidelines. -

Quality Control and Reliability Engineering 1152Au128 3 0 0 3
L T P C QUALITY CONTROL AND RELIABILITY ENGINEERING 1152AU128 3 0 0 3 1. Preamble This course provides the essentiality of SQC, sampling and reliability engineering. Study on various types of control charts, six sigma and process capability to help the students understand various quality control techniques. Reliability engineering focuses on the dependability, failure mode analysis, reliability prediction and management of a system 2. Pre-requisite NIL 3. Course Outcomes Upon the successful completion of the course, learners will be able to Level of learning CO Course Outcomes domain (Based on Nos. C01 Explain the basic concepts in Statistical Process Control K2 Apply statistical sampling to determine whether to accept or C02 K2 reject a production lot Predict lifecycle management of a product by applying C03 K2 reliability engineering techniques. C04 Analyze data to determine the cause of a failure K2 Estimate the reliability of a component by applying RDB, C05 K2 FMEA and Fault tree analysis. 4. Correlation with Programme Outcomes Cos PO1 PO2 PO3 PO4 PO5 PO6 PO7 PO8 PO9 PO10 PO11 PO12 PSO1 PSO2 CO1 H H H M L H M L CO2 H H H M L H H M CO3 H H H M L H L H CO4 H H H M L H M M CO5 H H H M L H L H H- High; M-Medium; L-Low 5. Course content UNIT I STATISTICAL QUALITY CONTROL L-9 Methods and Philosophy of Statistical Process Control - Control Charts for Variables and Attributes Cumulative Sum and Exponentially Weighted Moving Average Control Charts - Other SPC Techniques Process - Capability Analysis - Six Sigma Concept.