Granulomatous Lung Disease: How Pathologic Findings Add Value to Clinical And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pulmonary Pathology (Including Mediastinal)
ANNUAL MEETING ABSTRACTS 467A Pulmonary Pathology (including Mediastinal) 1849 Loss of p16INK4A Expression Predicts Worse Outcome in Thymic Carcinoma Scott W Aesif, Marie-Christine Aubry, Eunhee S Yi, Sarah M Jenkins, Grant Spears, Anja C Roden. Mayo Clinic, Rochester, MN. Background: In many human cancers, abnormalities in genes and proteins that regulate the cell cycle have been demonstrated. For instance, p16INK4A (p16), a tumor suppressor, inhibits cyclin D1-mediated phosphorylation of the retinoblastoma protein and ultimately leads to cell cycle blockade. In non-small cell lung cancer, low p16 expression has been associated with increased cyclin D1 expression and worse survival. In thymic carcinoma, studies showed loss of p16 expression in approximately two thirds of cases. However, the association of loss of p16 expression with cyclin D1 expression, prognosis, stage and clinical parameters has not been investigated in thymic carcinomas. Design: Thymic carcinomas (1963-2013) were reviewed by 3 thoracic pathologists who agreed on the diagnosis. Medical records were studied. Consecutive slides were stained for p16 and cyclin D1. Percent tumor cell staining (p16, cyclin D1) was scored as 0 (<1% or negative), 1+ (1-25% tumor cells staining), 2+ (26-50%), 3+ (51-75%), Conclusions: In this limited data set, we find that BMM cases with less than 50% and 4+ (>75%). Staining profiles were correlated with patient demographics, clinical sarcomatoid component have a survival advantage. Significant interobserver agreement presentation, pathologic staging (TNM and modified Masaoka), tumor subtype (WHO), is noted between two independent pathologists in sub-stratifying BMM cases. tumor size, and overall (OS) and disease free survival (DFS). -

Pulmonary Pathology Fellowship
PULMONARY PATHOLOGY FELLOWSHIP The Department of Pathology and Laboratory Medicine University of Calgary Calgary, Alberta Description and Objectives Pulmonary Pathology Fellowship Prepared: December 2011 PULMONARY PATHOLOGY FELLOWSHIP Description Fellowship Director: Margaret M. Kelly Fellowship purpose: • To train pathologists to understand and diagnose routine, complex and esoteric pathologic specimens from the lung, pleura and mediastinum • To train pathologists to effectively educate and communicate with medical students, trainees, other pathologists and clinicians regarding diseases of the lung, pleura and mediastinum • To advance the discipline of pulmonary pathology. The Pulmonary Pathology Fellowship Program will be administered under the auspices of the Division of Anatomic Pathology and will be mostly based at the Foothills Medical Centre. Curriculum: The Fellow is expected to be present in the department during laboratory working hours: Monday - Friday, 8:00 a.m. to 5:00.p.m. The curriculum consists of rotations through the Pulmonary Pathology Consult services (8 months), pediatric pathology (1 month) and research (3 months). Participation in the Pulmonary Pathology Consult service will include coverage of frozen section activities (of lung, pleura and mediastinal lesions), in collaboration with surgical pathology residents and faculty. It will also include observing various surgical techniques related to lung. This will include observing VATS, open lung resections, transbronchial biopsies and EBUS procedures. These will be co-ordinated with Dr. Gary Gelfand (head of thoracic surgery) and Dr. Alain Tremblay (head of interventional pulmonology). To supplement the “on the job” learning, the fellow will utilize Dr. Kelly’s pulmonary pathology teaching collection, relevant cases from the departmental teaching collection, participate in medical student teaching, and participate in regularly scheduled interdisciplinary conferences including a multi-institutional journal club. -

Tissue Pathways for Non-Neoplastic Thoracic Pathology March 2020
Tissue pathways for non-neoplastic thoracic pathology March 2020 Authors: Dr Irshad Soomro, Nottingham University Hospitals NHS Trust Dr Andrew Robinson, University Hospitals Birmingham NHS Foundation Trust Professor Andrew Nicholson, Royal Brompton Hospital and Harefield NHS Foundation Trust Unique document number G135 Document name Tissue pathways for non-neoplastic thoracic pathology Version number 2 Produced by Dr Irshad Soomro is a consultant histopathologist, Honorary Clinical Associate Professor and lead thoracic pathologist at Nottingham University Hospitals NHS Trust. Dr Andrew Robinson is a consultant histopathologist and lead thoracic pathologist at Birmingham Heartlands Hospital, University Hospitals Birmingham NHS Foundation Trust. Professor Andrew Nicholson is a consultant histopathologist at Royal Brompton Hospital and Harefield NHS Foundation Trust, London, and specialises in thoracic pathology. He is a member of the Pathology Committee and Staging and Prognostic Factors Committee of the International Association for the Study of Lung Cancer (IASLC). He has co-authored internationally agreed management guidelines for idiopathic pulmonary fibrosis (2018), and the 2013 update on classification of idiopathic interstitial pneumonias. Date active March 2020 Date for review March 2025 Comments This document supersedes the 2013 edition of Tissue pathways for non- neoplastic thoracic pathology. In accordance with the College’s pre-publications policy, this document was on the Royal College of Pathologists’ website for consultation from 7 November to 5 December 2019. Responses and authors’ comments are available to view on publication of the final document. Dr Brian Rous Clinical Lead for Guideline Review (Cellular Pathology) The Royal College of Pathologists 6 Alie Street, London E1 8QT Tel: 020 7451 6700 Fax: 020 7451 6701 Web: www.rcpath.org Registered charity in England and Wales, no. -
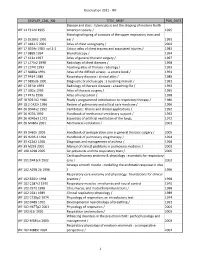
WF Blog.Xlsx
Deselection 2011 ‐ WF DISPLAY_CALL_NO TITLE_BRIEF PUB_DATE Disease and class : tuberculosis and the shaping of modern North WF 11 F312d 1995 American society / 1995 Histological typing of tumours of the upper respiratory tract and WF 15 S528h2 1991 ear / 1991 WF 17 A861.5 2003 Atlas of chest sonography / 2003 WF 17 B559c 1983 vol.1‐2 Colour atlas of chest trauma and associated injuries / 1983 WF 17 B869 1994 Bronchoscopy / 1994 WF 17 K13a 1997 Atlas of general thoracic surgery / 1997 WF 17 L274r2 1998 Radiology of chest diseases / 1998 WF 17 L274t 1993 Teaching atlas of thoracic radiology / 1993 WF 17 N886a 1991 Atlas of the difficult airway : a source book / 1991 WF 17 R434 1989 Respiratory diseases : clinical atlas / 1989 WF 17 S895d6 1991 Diagnostic bronchoscopy : a teaching manual / 1991 WF 17 S974r 1993 Radiology of thoracic diseases : a teaching file / 1993 WF 17 U82a 1995 Atlas of thoracic surgery / 1995 WF 17 Y47a 1998 Atlas of lung cancer / 1998 WF 18 B261b2 1980 Brady's programmed introduction to respiratory therapy / 1980 WF 18.2 C423r 1996 Review of pulmonary and critical care medicine / 1996 WF 26 D944v2 1992 Ventilators : theory and clinical applications / 1992 WF 26 H236 1992 Handbook of mechanical ventilatory support / 1992 WF 26 H945e3 1972 Essentials of artificial ventilation of the lungs, 1972 WF 26 M486a 2001 Mechanical ventilation / 2001 WF 39 D461h 2005 Handbook of perioperative care in general thoracic surgery / 2005 WF 39 H236.4 1994 Handbook of pulmonary drug therapy / 1994 WF 39 K22d2 1998 Diagnosis and management -

Spontaneous Pneumothorax As Unusual Presenting Symptom Of
Bellini et al. Journal of Cardiothoracic Surgery (2020) 15:310 https://doi.org/10.1186/s13019-020-01343-4 CASE REPORT Open Access Spontaneous pneumothorax as unusual presenting symptom of COVID-19 pneumonia: surgical management and pathological findings Roberto Bellini1†, Maria Chiara Salandini1*† , Serena Cuttin2, Stefania Mauro3, Paolo Scarpazza4 and Christian Cotsoglou1 Abstract Background: Spontaneous pneumothorax has been reported as a possibile complication of novel coronavirus associated pneumonia (COVID-19). We report two cases of COVID-19 patients who developed spontaeous and recurrent pneumothorax as a presenting symptom, treated with surgical procedure. An insight on pathological finding is given. Case presentation: Two patients presented to our hospital with spontaneous pneumothorax associated with Sars- Cov2 infection onset. After initial conservative treatment with chest drain, both patients had a recurrence of pneumothorax during COVI-19 disease, contralateral (patient 1) or ipsilateral (patient 2) and therefore underwent lung surgery with thoracoscopy and bullectomy. Intraoperative findings of COVID-19 pneumonia were parenchymal atelectasis and vascular congestion. Lung tissue was very frail and prone to bleeding. Histological examination showed interstitial infiltration of lymphocytes and plasma cells, as seen in non specific interstitial pneumonia, together with myo-intimal thicknening of vessels with blood extravasation and microthrombi. Conclusions: Although rarely, COVID-19 may present with spontaneous pneumothorax. Lung surgery for pneumothorax in COVID-19 patients can be safely and effectively performed when necessary. Keywords: Pneumothorax, COVID-19, Thoracoscopy, Case report, Bullectomy, Pathology Introduction Patients with COVID-19 can have mild symptoms On December 2019, a novel coronavirus-related pneu- such as fever, cough, fatigue and smell and taste dys- monia has been first reported in the city of Wuhan, function, or more serious and rapidly progressing re- China [1]. -

Pulmonary Pathology (Including Mediastinal)
ANNUAL MEETING ABSTRACTS 471A Results: We show identical molecular signature in the original leukemic blasts and the 1895 Norovirus Infection in Pediatric Small Intestine Allografts: A subsequent histiocytic neoplasms in two pediatric patients with TALL. Using FISH, one Clinicopathological Study of a Cohort of 23 Patients case had the same biallelic deletion of the CDKN2A (TP16) locus on 9p21, while using Wei Xu, Stuart Kaufman, Joeffrey Chahine, Brandi Higgins, Nada Yazigi, Cal next generation sequencing, the other had the same G13D mutation in the NRAS gene. Matsumoto, Khalid Khan, Bhaskar VS Kallakury. Medstar Georgetown Univeristy Conclusions: This confirms a common clonal origin and highlights the need to perform Hospital, Washington, DC. appropriate molecular studies in such patients to better understand the mechanism Background: Human Norovirus in the family Caliciviridae is a major cause of and etiology behind histiocytic neoplasm seemingly born out of other hematopoietic epidemic gastroenteritis. Norovirus infections are typically acute and self-limited. malignancies. However, Norovirus infection, the most frequent cause of acute pediatric gastroenteritis in the era of Rotavirus vaccine, produces a prolonged and chronic diarrhea in immunocompromised host, particularly in pediatric small intestine transplant recipients. 1893 Assessment of PD-L1 Expression in Pediatric High-Grade This study aimed to characterize histological and immunohistochemical features of Gliomas Mariona Suñol, Iban Aldecoa, Ofelia Cruz, Angel Montero, Eva Rodríguez, Teresa small bowl allograft biopsies before and after Novovirus infection. Ribalta. Sant Joan de Déu Barcelona Children’s Hospital, Barcelona, Spain. Design: We retrospectively reviewed H&E slides and performed IHC staining for Background: Programmed death-ligand 1 (PD-L1) is an immune-inhibitory receptor proliferation index (Ki67), apoptosis (Caspase 3), T lymphocytes (CD3) on pre and expressed in some tumors, including high-grade gliomas (HGG). -

Essentials of Lung Tumor Cytology
ESSENTIALS OF LUNG TUMOR CYTOLOGY Gia-Khanh Nguyen 2008 1 ESSENTIALS OF LUNG TUMOR CYTOLOGY Gia-Khanh Nguyen, M.D. Professor Emeritus Laboratory Medicine and Pathology University Of Alberta Edmonton, Alberta, Canada Copyright by Gia-Khanh Nguyen Revised first edition, 2008 First edition, 2007. All rights reserved. This book was legally deposited at the Library and Archives Canada. ISNB: 0-9780929-0-2 2 TABLES OF CONTENTS Table of contents 3 Preface 4 Dedication 5 Acknowledgement and Related material 6 Key to abbreviations 7 Chapter 1: Cytologic investigations of lung tumors 8 Chapter 2: Usual lung cancers 18 Chapter 3: Neuroendocrine carcinomas 38 Chapter 4: Other primary tumors and tumorlike lesions 49 Chapter 5: Metastatic cancers 65 Chapter 6: Pleural tumors 77 PREFACE Cytology plays a very important role in the diagnosis of lung cancers. The monograph “Essentials of Lung Tumor Cytology “ is the result of my experience gained in over 20 years of active involvement in the cytodiagnosis of lung tumors at the University of Alberta Hospital, Edmonton, Alberta, Canada. It is written for practicing pathologists in community hospitals, residents in pathology and cytotechnologists who are interested in making safe and accurate cytodiagnoses of important tumors of the lung and pleura. The text is concise and illustrations are abundant. Several of histologic images are included for cytohistologic correlation. In the first edition of the monograph (2007), cytodiagnostic criteria of lung tumors were presented. In this revised edition, immunocytochemical features of lung tumor cells that are important for tumor typing and differential diagnosis are stressed. A number of important references are listed at the end of each chapter for further consultation. -

Mskcc@Uscap 2019
MSKCC@USCAP 2019 Gaylord National Resort & Convention Center Saturday March 16 Saturday Evening Companion Meeting 7:40 pm (International Society of Urological Pathology): The Importance of Pathologic Evaluation in Active Surveillance of Small Renal Masses – Ying-Bei Chen [National Harbor 4/5] 7:40 pm (Pancreatobiliary Pathology Society): Pancreatic Neuroendocrine Neoplasms – Landscape and Horizon – Laura Tang [Maryland Ballroom B] 8:10 pm (Pulmonary Pathology Society): Lung Adenocarcinoma: Emerging Histologic Patterns – William Travis [National Harbor 11] 8:50 pm (Pulmonary Pathology Society): Update on SMARCB4 Deficient Lung Tumors – Natasha Rekhtman [National Harbor 11] 8:50 pm (Association for Pathology Informatics): Robotic Telecytology for Remote Diagnosis – S. Joseph Sirintrapun [National Harbor 10] Sunday March 17 Sunday Morning Companion Meeting 9:00 am (Arthur Purdy Stout Society of Surgical Pathologists): The Disease Doesn’t Know Your Age: Adults and Children with the “Wrong Affliction”? GU Pathology – Victor Reuter [Maryland Ballroom] 11:00 am (International Society of Urological Pathology Special Conference on Molecular Pathology of Urogenital Cancers – Molecular Pathology Kidney Cancer Working Group): Eosinophilic solid and cystic renal cell carcinoma and renal tumors associated with TSC-mTOR pathways; distinguishing papillary renal cell carcinoma and mucinous tubular spindle cell carcinoma – Ying-Bei Chen [MGM Grand Hotel] Sunday Afternoon Companion Meeting 2:50 pm (Genitourinary Pathology Society): Pathology and Molecular