Convention on the Conservation of Migratory Species of Wild Animals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

English Version of the Mediterranean Edition of the Handbook on Effective Labour Migration Policies, Edition of the Handbook on Effective Labour Migration Policies
Activity Report June 2007 – May 2008 Office of the Co-ordinator of OSCE Economic and Environmental Activities osce.org/eea Organization for Security and Co-operation in Europe Activity Report June 2007 – May 2008 Office of the Co-ordinator of OSCE Economic and Environmental Activities Organization for Security and Co-operation in Europe PUBLISHED BY Office of the Co-ordinator of OSCE Economic and Environmental Activities OSCE Secretariat Wallnerstrasse 6, A-1010 Vienna, Austria Tel: +43 1 514 36 6151 Fax: +43 1 514 36 6251 E-mail: [email protected] Vienna, May 2008 osce.org/eea This is not a consensus document. EDITORS Roel Janssens, Sergey Kostelyanyets, Gabriel Leonte, Kilian Strauss, Alexey Stukalo. DESIGN AND PRINTING Phoenix Design Aid A/S, Denmark. ISO 14001/ISO 9000 certified and EMAS-approved. Produced on 100% recycled paper (without chlorine) with vegetable-based inks. The printed matter is recyclable. PHOTOS All pictures unless indicated otherwise: OSCE Front cover pictures: Shamil Zhumatov and OSCE Table of Contents 1. INTRODUCTION BY THE CO-ORDINATOR OF OSCE ECONOMIC AND ENVIRONMENTAL ACTIVITIES 05 2. CURRENT ISSUES AND RECENT DEVELOPMENTS IN THE ECONOMIC AND ENVIRONMENTAL DIMENSION 07 2.1 Political dialogue on topical Economical and Environmental issues 07 2.2 Enhancing synergies between Vienna and the OSCE field presences 10 3. THE 16TH ECONOMIC AND ENVIRONMENTAL FORUM 12 3.1 Helsinki Preparatory Conference 12 3.2 Vienna Forum 13 3.3 Ashgabad Preparatory Conference 14 4. GOOD GOVERNANCE: COMBATING CORRUPTION, MONEY LAUNDERING AND TERRORIST FINANCING 16 4.1 Promoting transparency and combating corruption 16 4.2 Strengthening of legislation and promotion of international standards 18 4.3 Activities aimed at combating money laundering and the financing of terrorism 19 5. -

Academia De Ştiinţe a Moldovei Institutul De Zoologie
ACADEMIA DE ŞTIINŢE A MOLDOVEI INSTITUTUL DE ZOOLOGIE APROB Directorul IZ AŞM, academician Ion Toderaş L.Ş „ _______”________________2015__ RAPORT DE AUTOEVALUARE A INSTITUTULUI DE ZOOLOGIE AL ACADEMIEI DE ŞTIINŢE A MOLDOVEI anii 2010-2014 PROFILUL Sistematica, evoluția și valorificarea sustenabilă a diversității lumii animale, monitoringul ecosistemelor acvatice și terestre Aprobat la şedinţa Consiliului ştiinţific al Institutului de Zoologie al AŞM din 22 octombrie 2015 Chişinău 2015 1 © Prin prezenta Institutul de Zoologie al AŞM anunţă despre acordul amplasării datelor despre acreditarea instituţiei precum şi a profilului respectiv pe site-ul CNAA 2 CUPRINS Nr.d/o Conţinut Numărul pag. 1. Date generale 7 1.1. Istoricul organizaţiei 7 1.2. Statutul juridic actual şi subordonarea sectorială 9 1.3. Misiunea organizaţiei 9 1.4. Elementele cheie ale programului managerial, expuse la 10 concursul de suplinire a funcţiei vacante de director al organizaţiei 1.5. Obiectivele realizate ale proiectului managerial 11 2. Capacitatea instituţională şi resursele 13 2.1. Cadrul tematic şi instituţional de cercetare 13 2.1.1. Structura instituţională 13 2.1.2. Drecţiile principale de cercetare ale organizaţiei 15 2.1.3. Proiecte instituţionale 17 2.1.4. Proiectele din cadrul programelor de stat 32 2.1.5. Proiecte de cercetare internaţionale 34 2.1.6. Proiecte pentru tineri cercetători 38 2.1.7. Proiecte pentru procurarea utilajului 42 2.1.8. Granturi internaționale 42 2.1.9. Proiecte finanțate de Fondul Ecologic Național 44 2.1.10 Contractele economice 48 2.2. Personalul uman 54 2.2.1. Componenţa nominală a personalului de conducere 54 2.2.2. -
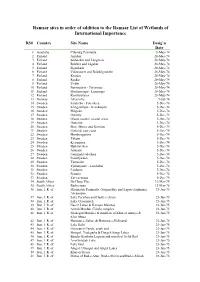
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

1. World Heritage Property Data 2. Statement of Outstanding Universal Value
Periodic Report - Second Cycle Section II-Danube Delta 1. World Heritage Property Data 2. Statement of Outstanding Universal Value 1.1 - Name of World Heritage Property 2.1 - Statement of Outstanding Universal Value / Danube Delta Statement of Significance Comment 1.2 - World Heritage Property Details At the time of inscription, IUCN carried out an evaluation of the State(s) Party(ies) authenticity and/or integrity of the property. The World Heritage Committee approved at the date of inscription a Romania Statement of Significance for the site, which define the Type of Property Outstanding Universal Value. The Statement of Significance natural for the site still adequately define and reflect the Outstanding Identification Number Universal Value of the site. Details of the evaluation: The Danube Delta Biosphere Reserve is the largest continuous 588 marshland in Europe and the second largest delta (the Volga Year of inscription on the World Heritage List delta being the largest), which includes the greatest stretch of 1991 reedbeds in the world. Over 300 species of birds have been recorded, of which over 176 species breed. The delta is very important for fish, with 85 fresh water species present. The 1.3 - Geographic Information Table Danube Delta is a remarkable alluvial feature constituting Name Coordinates Property Buffer Total Inscription critical habitats for migratory birds and other animals. It is the (latitude/longitude) (ha) zone (ha) year (ha) major remaining wetland on the flyway between central and Danube 45.083 / 29.5 312440 0 312440 1991 eastern Europe and the Mediterranean and Middle East and Delta Africa. It is exceptional for its contiguity of wetlands Total (ha) 312440 0 312440 ecosystems and currently supports endangered flora and fauna. -

Școala Doctorală Științe Biologice Publicații Relevante Ale Conducătorilor De Doctorat (Ultimii 5 Ani)
Școala doctorală științe biologice Publicații relevante ale conducătorilor de doctorat (ultimii 5 ani) FURDUI TEODOR, academician, dr. hab. șt. biol., prof. univ. 1. Monografii 1. ФУРДУЙ Ф.И., КРАСОЧКО П.А., ШЕЙКО И.П., и др. Физиологические основы проявления стрессов и пути их коррекции в промышленном животноводстве. Горки: БГСХА, 2013. Ч.1, 492 с. ISBN 978-985-467-451-3. 2. ФУРДУЙ Ф.И., КРАСОЧКО П.А., ШЕЙКО И.П., и др. Физиологические основы проявления стрессов и пути их коррекции в промышленном животноводстве. Горки: БГСХА, 2013. Ч.2, 564с. ISBN 978-985-467-452-0. 3. ФУРДУЙ Ф.И., ЧОКИНЭ В.К., ФУРДУЙ В.Ф. Преждевременная общебиологическая деградация современного общества, регулирование его воспроизводства, саногенное питание и пути их решения – важнейшие межгосударственные проблемы. Итоги науки. Том 3, Избранные труды Международного симпозиума по фундаментальным и прикладным проблемам науки. М.: РАН, Глава 6. 2014, c.85-112. 4. FURDUI T., CIOCHINĂ V. Sanocreatologia și haltere. În: Tudor Casapu, eternul campion. Colecția Personalități notorii. Chișinău: Institutul de Studii Enciclopedice, 2015, pp. 77-81. ISBN 978-9975- 3044-3-6 5. ФУРДУЙ Ф.И., ЧОКИНЭ В.К., ФУРДУЙ В.Ф., ГЛИЖИН А.Г., ВРАБИЕ В.Г. ШЕПТИЦКИЙ В.А. Трактат о научных и практических основах санокреатологии. Том 1. Проблема здоровья. Санокреатология. Потребность общества в ее развитии. Chișinău: Tipografia AȘM, 2016, 228 p. ISBN 978-9975-62-400-8. 2. Articole în diferite reviste ştiinţifice 2.1. în reviste internaţionale cotate ISI şi SCOPUS 1. FURDUI F.I., SHEPTITSKII V.A., CEBAN L.N. Features of monosaccharide absorption modification in the small intestine by a high carbohydrate diet in early postnatal ontogenesis. -

Black Sea-Caspian Steppe: Natural Conditions 20 1.1 the Great Steppe
The Pechenegs: Nomads in the Political and Cultural Landscape of Medieval Europe East Central and Eastern Europe in the Middle Ages, 450–1450 General Editors Florin Curta and Dušan Zupka volume 74 The titles published in this series are listed at brill.com/ecee The Pechenegs: Nomads in the Political and Cultural Landscape of Medieval Europe By Aleksander Paroń Translated by Thomas Anessi LEIDEN | BOSTON This is an open access title distributed under the terms of the CC BY-NC-ND 4.0 license, which permits any non-commercial use, distribution, and reproduction in any medium, provided no alterations are made and the original author(s) and source are credited. Further information and the complete license text can be found at https://creativecommons.org/licenses/by-nc-nd/4.0/ The terms of the CC license apply only to the original material. The use of material from other sources (indicated by a reference) such as diagrams, illustrations, photos and text samples may require further permission from the respective copyright holder. Publication of the presented monograph has been subsidized by the Polish Ministry of Science and Higher Education within the National Programme for the Development of Humanities, Modul Universalia 2.1. Research grant no. 0046/NPRH/H21/84/2017. National Programme for the Development of Humanities Cover illustration: Pechenegs slaughter prince Sviatoslav Igorevich and his “Scythians”. The Madrid manuscript of the Synopsis of Histories by John Skylitzes. Miniature 445, 175r, top. From Wikimedia Commons, the free media repository. Proofreading by Philip E. Steele The Library of Congress Cataloging-in-Publication Data is available online at http://catalog.loc.gov LC record available at http://catalog.loc.gov/2021015848 Typeface for the Latin, Greek, and Cyrillic scripts: “Brill”. -

Umbra Krameri
4. В.Зенкин, Е.Рязанцева, О.Лосев „Полиморфизм мышечных эстераз и анализ популяционной структуры обыкновенной и капской ставрид шельфа западной Африки” (Биохимическая и популяционная генетика рыб), Ленинград,1979. c.94 5. В.Кирпичников “Генетика и селекция рыб”, Ленинград, 1987. pag.519 6. Л.Смирнов “Сравнительная оценка белковых спектров печени и мускулатуры рыб, птиц и млекопитающих, получаемых методом диск-электрофореза в полиакриламидном геле” (Сравнительная биохимия рыб и их гельминтов) Петрoзаводск, 1977. Pag.85 7. Л.Шарт, Ю.Илясов „О типах трансферpинов и эстераз у производителей карпа селекционируемых нa устойчивость к краснухе”, (Биохимическая и популяционная генетика рыб), Ленинград, 1979. Pag.147 TWO NEW CNIDOSPOREAN SPECIES (CNIDOSPORA: SPHAERO- SPORIDAE, MYXOBOLIDAE), PARASITES OF THE EUROPEAN MUDMIN- NOW (UMBRA KRAMERI) FROM LOWER DNIESTER RIVER Alexander Moshu, Ilya Trombitsky* Institute of Zoology, Academy of the Sciences of Moldova; *Fisheries Research Station Introduction The European mudminnow – Umbra krameri Walbaum, 1792 (Esociformes: Umbridae) from the zoogeographical point of view is as a relict endemic of the Danube and Lower Dniester River basins and of the Black Sea region of Ponto-Aralo-Caspian province of Holarctic [1]. At present this freshwater fish is highly rare and figures in Lists of the Red Books of the World, Europe, Romania, Republic of Moldova and Ukraine as an especially threatened species [13]. The analysis of the collected material showed, that parasitic fauna of U.krameri is rich and diverse (about 45 species of different taxonomical groups) and is included on the whole with the common and widely-distributed species of the Dniester River and other waterbodies of the region. Among them only nine species seem to be more or less specific for this fish and pike (Esox lucius L., 1758) [28]. -

ND2 As an Additional Genetic Marker to Improve Identification of Diving Ducks Involved in Bird Strikes Sarah A
Human–Wildlife Interactions 14(3):365–375, Winter 2020 • digitalcommons.usu.edu/hwi ND2 as an additional genetic marker to improve identification of diving ducks involved in bird strikes Sarah A. M. Luttrell, Division of Birds, National Museum of Natural History, Smithsonian Institution, 10th and Constitution Ave. NW, Washington, DC 20560, USA [email protected] Sergei Drovetski,1 Division of Birds, National Museum of Natural History, Smithsonian Institu- tion, 10th and Constitution Ave. NW, Washington, DC 20560, USA Nor Faridah Dahlan, Division of Birds, National Museum of Natural History, Smithsonian Institution, 10th and Constitution Ave. NW, Washington, DC 20560, USA Damani Eubanks,2 Division of Birds, National Museum of Natural History, Smithsonian Institu- tion, 10th and Constitution Ave. NW, Washington, DC 20560, USA Carla J. Dove, Division of Birds, National Museum of Natural History, Smithsonian Institution, 10th and Constitution Ave. NW, Washington, DC 20560, USA Abstract: Knowing the exact species of birds involved in damaging collisions with aircraft (bird strikes) is paramount to managing and preventing these types of human–wildlife conflicts. While a standard genetic marker, or DNA barcode (mitochondrial DNA gene cytochrome-c oxidase 1, or CO1), can reliably identify most avian species, this marker cannot distinguish among some closely related species. Diving ducks within the genus Aythya are an example of congeneric waterfowl involved in bird strikes where several species pairs cannot be reliably identified with the standard DNA barcode. Here, we describe methods for using an additional genetic marker (mitochondrial DNA gene NADH dehydrogenase subunit 2, or ND2) for identification of 9Aythya spp. Gene-specific phylogenetic trees and genetic distances among taxa reveal that ND2 is more effective than CO1 at genetic identification of diving ducks studied here. -

The Status of Ferruginous Duck Aythya Nyroca Breeding and Wintering in China
116 The status of Ferruginous Duck Aythya nyroca breeding and wintering in China ZHAO XUMAO1,2 & ROLLER MAMING1* 1Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, Urumqi, Xinjiang 830011, P.R. China. 2University of Chinese Academy of Sciences, Beijing 100049, P.R. China. *Correspondence author. E-mail: [email protected] Abstract Analysis of data published in the China Bird Report (CBR) and other literature between 1979 and 2013, along with our own field observations, found that the Ferruginous Duck Aythya nyroca now occurs throughout most of China. This contrasts with the situation in 1979, when the species was restricted to a few areas in the west of the country. Ferruginous Duck is predominately a winter visitor to China; up to 850 birds have been counted in Yunnan Province, 4,000 in Sichuan Province, and the wintering population is estimated at 6,000–8,000 individuals. In summer, the breeding population is estimated at 3,000–4,000 individuals (1,500–2,000 pairs), with highest concentrations in Xinjiang (c. 900–1,700 individuals) and Inner Mongolia (750–1,400 individuals). In China, females lay 6–11 eggs, which hatch in early June. The present population in China is likely to have spread from neighbouring central Asia (Kazakhstan, Kyrgyzstan or Mongolia). Since 1980, the climate had gradually changed from warm-dry conditions to a wetter climate in northwest China, making it a more suitable breeding area for this species and providing corridors for their expansion to the east. If the present trend continues, southern China could in the near future become an important wintering area for a duck species that is now increasingly common elsewhere in China. -
Hydrocharis Morsus-Ranae Global
FULL ACCOUNT FOR: Hydrocharis morsus-ranae Hydrocharis morsus-ranae System: Terrestrial Kingdom Phylum Class Order Family Plantae Magnoliophyta Liliopsida Hydrocharitales Hydrocharitaceae Common name common frogbit (English), European frog's-bit (English) Synonym Similar species Limnobium spongia Summary Hydrocharis morsus-ranae is a free-floating herbaceous annual aquatic that can reach 20cm in length. It does well in calm open waters, and can be found in marshes, ditches and swamps. H. morsus-ranae produces dense floating mat of vegetation which restrict available light, dissolved gases, and nutrients. This species displaces native flora and is perhaps impacting the fauna. There is currently no management information available but there is currently a study that started in 2003 and will go through 2005 that is looking into and researching methods of controlling H. morsus-ranae on the Great Lakes and St. Lawrence River. view this species on IUCN Red List Global Invasive Species Database (GISD) 2021. Species profile Hydrocharis morsus- Pag. 1 ranae. Available from: http://www.iucngisd.org/gisd/species.php?sc=862 [Accessed 05 October 2021] FULL ACCOUNT FOR: Hydrocharis morsus-ranae Species Description IPANE (2001) states that, \"H. morsus-ranae is an herbaceous, annual aquatic that can reach 20cm in length. The plant is free-floating. The leaves of this plant are usually floating, but if the vegetation is dense enough, they can be emergent. The leathery, glabrous leaves are cordate- orbicular in shape and measure 1.2-6cm in length and in width. The lower leaf surfaces are often dark purple in colour. H. morsus-ranae is a dioecious plant. -

Diurnal Time-Activity Budgets in Wintering Ferruginous Pochard Aythya Nyroca in Tanguar Haor, Bangladesh
FORKTAIL 20 (2004): 25–27 Diurnal time-activity budgets in wintering Ferruginous Pochard Aythya nyroca in Tanguar Haor, Bangladesh SABIR BIN MUZAFFAR Diurnal time-activity budgets were quantified for Ferruginous Pochard Aythya nyroca wintering in Tanguar Haor, Bangladesh. Individuals spent most time resting (60%), with less time spent feeding (17%), preening (14%) and swimming (9%). The time spent feeding was generally lower than for other Aythya species in winter, perhaps because Ferruginous Pochard feed preferentially at night. Human disturbance during the day may be a significant factor driving this. INTRODUCTION STUDY AREA The Ferruginous Pochard Aythya nyroca is widely The Haor basin is located in the north-eastern region distributed in Europe, Asia and Africa, but it has of Bangladesh. It contains the floodplains of the undergone declines in its populations and changes in Meghna river tributaries and it is characterised by distribution over the past few decades (Ali and Ripley numerous shallow water bodies known locally as beels, 1978, Perennou et al. 1994, Callaghan 1997, Lopez which coalesce in the wet season to form larger water and Mundkur 1997, Grimmett et al. 1999, Islam 2003, bodies (Rashid 1977, NERP 1993, Geisen et al. 2000). Robinson and Hughes 2003a,b). The primary reasons Tanguar Haor is one of the most important wetland for its decline are habitat degradation and loss and areas located near the northern reaches of the Haor hunting for local consumption (Callaghan 1997, basin (NERP 1993, Geisen et al. 2000). With a total Robinson and Hughes 2003a). The species is a winter area of 9,527 ha it is among the least disturbed of water visitor to the Indian subcontinent, where pressures on bodies in the area (NCSIP-1 2001a). -

European Frogbit (Hydrocharis Morsus-Ranae) Invasion Facilitated by Non- Native Cattails (Typha) in the Laurentian Great Lakes
JGLR-01497; No. of pages: 9; 4C: Journal of Great Lakes Research xxx (xxxx) xxx Contents lists available at ScienceDirect Journal of Great Lakes Research journal homepage: www.elsevier.com/locate/jglr European frogbit (Hydrocharis morsus-ranae) invasion facilitated by non- native cattails (Typha) in the Laurentian Great Lakes Andrew M. Monks a,⁎, Shane C. Lishawa a, Kathryn C. Wellons b, Dennis A. Albert b, Brad Mudrzynski c, Douglas A. Wilcox d a Institute of Environmental Sustainability, Loyola University Chicago, 1032 W. Sheridan Rd Chicago, IL 60660, USA b Department of Horticulture, Oregon State University, Agricultural and Life Sciences 4017, Corvallis, OR 97331, USA c Genesee County Soil and Water Conservation District, 29 Liberty St, Batavia, NY 14020, USA d Department of Environmental Science and Ecology, State University of New York: Brockport, Lennon Hall 108 B, Brockport, NY 14420, USA article info abstract Article history: Plant-to-plant facilitation is important in structuring communities, particularly in ecosystems with high levels of Received 22 March 2019 natural disturbance, where a species may ameliorate an environmental stressor, allowing colonization by another Accepted 1 July 2019 species. Increasingly, facilitation is recognized as an important factor in invasion biology. In coastal wetlands, Available online xxxx non-native emergent macrophytes reduce wind and wave action, potentially facilitating invasion by floating plants. We tested this hypothesis with the aquatic invasive species European frogbit (Hydrocharis morsus- Communicated by Anett Trebitz ranae; EFB), a small floating plant, and invasive cattail (Typha spp.), a dominant emergent, by comparing logistic Keywords: models of Great Lakes-wide plant community data to determine which plant and environmental variables European frogbit exerted the greatest influence on EFB distribution at multiple scales.