'Mechanism of Mpemba Effect'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table of Contents (Online, Part 1)
TM PERIODICALS PHYSICALREVIEWE Postmaster send address changes to: For editorial and subscription correspondence, please see inside front cover APS Subscription Services (ISSN: 2470-0045) P.O. Box 41 Annapolis Junction, MD 20701 THIRD SERIES, VOLUME 101, NUMBER 5 CONTENTS MAY 2020 PARTS A AND B The Table of Contents is a total listing of Parts A and B. Part A consists of pages 050101(R)–052320, and Part B pages 052401–059902(E). PART A RAPID COMMUNICATIONS Statistical Physics Measurability of nonequilibrium thermodynamics in terms of the Hamiltonian of mean force (6 pages) ......... 050101(R) Philipp Strasberg and Massimiliano Esposito Nonlinear Dynamics and Chaos Observation of accelerating solitary wavepackets (6 pages) ............................................... 050201(R) Georgi Gary Rozenman, Lev Shemer, and Ady Arie Networks and Complex Systems Kinetic roughening of the urban skyline (5 pages) ....................................................... 050301(R) Sara Najem, Alaa Krayem, Tapio Ala-Nissila, and Martin Grant Liquid Crystals Phase transitions of heliconical smectic-C and heliconical nematic phases in banana-shaped liquid crystals (4 pages) ................................................................................... 050701(R) Akihiko Matsuyama Granular Materials Two mechanisms of momentum transfer in granular flows (5 pages) ........................................ 050901(R) Matthew Macaulay and Pierre Rognon Direct measurement of force configurational entropy in jamming (5 pages) .................................. 050902(R) James D. Sartor and Eric I. Corwin Plasma Physics X-ray heating and electron temperature of laboratory photoionized plasmas (6 pages)......................... 051201(R) R. C. Mancini, T. E. Lockard, D. C. Mayes, I. M. Hall, G. P. Loisel, J. E. Bailey, G. A. Rochau, J. Abdallah, Jr., I. E. Golovkin, and D. Liedahl Copyright 2020 by American Physical Society (Continued) 2470-0045(202005)101:5:A;1-8 The editors and referees of PRE find these papers to be of particular interest, importance, or clarity. -
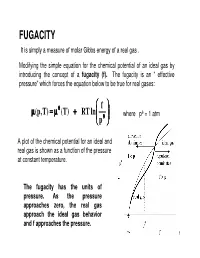
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

A Study of the Equilibrium Diagrams of the Systems, Benzene-Toluene and Benzene-Ethylbenzene
Loyola University Chicago Loyola eCommons Master's Theses Theses and Dissertations 1940 A Study of the Equilibrium Diagrams of the Systems, Benzene- Toluene and Benzene-Ethylbenzene John B. Mullen Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_theses Part of the Chemistry Commons Recommended Citation Mullen, John B., "A Study of the Equilibrium Diagrams of the Systems, Benzene-Toluene and Benzene- Ethylbenzene" (1940). Master's Theses. 299. https://ecommons.luc.edu/luc_theses/299 This Thesis is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Master's Theses by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1940 John B. Mullen A S'l'IJDY OF THE EQUTI.IBRIUM DIAGRAMS OF THE SYSTEMS, BENZENE-TOLUENE AND BENZENE-~ENZENE By John B. Mullen Presented in partial :f'u.J.tilment at the requirements :f'or the degree o:f' Master o:f' Science, Loyola University, 1940. T.ABLE OF CONTENTS Page Acknowledgment 1i Vita iii Introduction 1 I. Review of Literature 2 II. Apparatus and its Calibration 3 III. Procedure am Technique 6 IV. Observations on the System Benzene-Toluene 9 V. Observations on the System Benzene-Ethylbenzene 19 VI. Equilibrium Diagrams of the Systems 30 Recommendations for Future Work 34 Bibliography 35 ACKNOWLEOOMENT The au1hor wishes to acknowledge with thanks the invaluable assistance, suggestions, and cooperation offered by Dr. -

Liquid Pre-Freezing Percolation Transition to Equilibrium Crystal-In-Liquid Mesophase
http://www.scirp.org/journal/ns Natural Science, 2018, Vol. 10, (No. 7), pp: 247-262 Liquid Pre-Freezing Percolation Transition to Equilibrium Crystal-in-Liquid Mesophase Leslie V. Woodcock Department of Physics, University of Algarve, Faro, Portugal Correspondence to: Leslie V. Woodcock, Keywords: Liquid State, Percolation, Phase Transition, Pre-Freezing Mesophase Received: May 25, 2018 Accepted: June 23, 2018 Published: July 23, 2018 Copyright © 2018 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY 4.0). http://creativecommons.org/licenses/by/4.0/ Open Access ABSTRACT Pre-freezing anomalies are explained by a percolation transition that delineates the exis- tence of a pure equilibrium liquid state above the temperature of 1st-order freezing to the stable crystal phase. The precursor to percolation transitions are hetero-phase fluctuations that give rise to molecular clusters of an otherwise unstable state in the stable host phase. In-keeping with the Ostwald’s step rule, clusters of a crystalline state, closest in stability to the liquid, are the predominant structures in pre-freezing hetero-phase fluctuations. Evi- dence from changes in properties that depend upon density and energy fluctuations suggests embryonic nano-crystallites diverge in size and space at a percolation threshold, whence a colloidal-like equilibrium is stabilized by negative surface tension. Below this transition temperature, both crystal and liquid states percolate the phase volume in an equilibrium state of dispersed coexistence. We obtain a preliminary estimate of the prefreezing percola- tion line for water determined from higher-order discontinuities in Gibbs energy that de- rivatives the isothermal rigidity [(dp/dρ)T] and isochoric heat capacity [(dU/dT)v] respec- tively. -

Phase Diagrams
Module-07 Phase Diagrams Contents 1) Equilibrium phase diagrams, Particle strengthening by precipitation and precipitation reactions 2) Kinetics of nucleation and growth 3) The iron-carbon system, phase transformations 4) Transformation rate effects and TTT diagrams, Microstructure and property changes in iron- carbon system Mixtures – Solutions – Phases Almost all materials have more than one phase in them. Thus engineering materials attain their special properties. Macroscopic basic unit of a material is called component. It refers to a independent chemical species. The components of a system may be elements, ions or compounds. A phase can be defined as a homogeneous portion of a system that has uniform physical and chemical characteristics i.e. it is a physically distinct from other phases, chemically homogeneous and mechanically separable portion of a system. A component can exist in many phases. E.g.: Water exists as ice, liquid water, and water vapor. Carbon exists as graphite and diamond. Mixtures – Solutions – Phases (contd…) When two phases are present in a system, it is not necessary that there be a difference in both physical and chemical properties; a disparity in one or the other set of properties is sufficient. A solution (liquid or solid) is phase with more than one component; a mixture is a material with more than one phase. Solute (minor component of two in a solution) does not change the structural pattern of the solvent, and the composition of any solution can be varied. In mixtures, there are different phases, each with its own atomic arrangement. It is possible to have a mixture of two different solutions! Gibbs phase rule In a system under a set of conditions, number of phases (P) exist can be related to the number of components (C) and degrees of freedom (F) by Gibbs phase rule. -

When the Hotter Cools More Quickly: Mpemba Effect in Granular Fluids
week ending PRL 119, 148001 (2017) PHYSICAL REVIEW LETTERS 6 OCTOBER 2017 When the Hotter Cools More Quickly: Mpemba Effect in Granular Fluids Antonio Lasanta,1,2 Francisco Vega Reyes,2 Antonio Prados,3 and Andrés Santos2 1Gregorio Millán Institute of Fluid Dynamics, Nanoscience and Industrial Mathematics, Department of Materials Science and Engineering and Chemical Engineering, Universidad Carlos III de Madrid, 28911 Leganés, Spain 2Departamento de Física and Instituto de Computación Científica Avanzada (ICCAEx), Universidad de Extremadura, 06006 Badajoz, Spain 3Física Teórica, Universidad de Sevilla, Apartado de Correos 1065, 41080 Sevilla, Spain (Received 16 November 2016; revised manuscript received 20 March 2017; published 4 October 2017) Under certain conditions, two samples of fluid at different initial temperatures present a counterintuitive behavior known as the Mpemba effect: it is the hotter system that cools sooner. Here, we show that the Mpemba effect is present in granular fluids, both in uniformly heated and in freely cooling systems. In both cases, the system remains homogeneous, and no phase transition is present. Analytical quantitative predictions are given for how differently the system must be initially prepared to observe the Mpemba effect, the theoretical predictions being confirmed by both molecular dynamics and Monte Carlo simulations. Possible implications of our analysis for other systems are also discussed. DOI: 10.1103/PhysRevLett.119.148001 Let us consider two identical beakers of water, initially at In a general physical system, the study of the ME implies two different temperatures, put in contact with a thermal finding those additional variables that control the temper- reservoir at subzero (on the Celsius scale) temperature. -

How Does Water Freeze 12 August 2012 16:34
How does water freeze 12 August 2012 16:34 Water is an unique substance that consists of many unusual properties. The two elements making up water are Hydrogen and Oxygen. There are two Hydrogen atoms per Oxygen atom. Hence the Molecular Formula of water is H2O. This makes water a polar compound and soluble with many substances. As hydrogen and oxygen are both non-metals, covalent bonding is used to form the compound. In covalent bonding the more electronegative (ability to attract electrons) atom has a slightly negative charge and the less electronegative is slightly positively charged. Polar molecules are attracted to one another by dipole interaction. Hydrogen has an electronegativity of 2.1 and Oxygen is 3.5. Therefore the difference in electronegativity is 1.4. The negative end of one molecule of water is attracted to the positive end of another. This results in hydrogen bonding. Hydrogen bonding occurs when the hydrogen bonds with a highly electronegative element e.g. O, F, N. The Hydrogen (slightly positive charge) is attracted to the lone pair of electrons in the nearby atom of Oxygen (the highly electronegative element). This Hydrogen bond has about 5% of the strength of a standard covalent bond. Hydrogen bonds are the strongest of all intermolecular forces. Hydrogen bonding in water is the only reason why it has such a high specific heat. Specific heat can be defined as the amount of heat required to change a unit mass ( such as a mole) of a substance by one degree in temperature. Hydrogen bonding weakens as the temperature rises, therefore much of the energy is used into breaking hydrogen bonds instead of raising the temperature. -

Neutron Star Cooling and Discuss the Main Issue: What We Can Learn About the Internal Structure of Neutron Stars by Confronting Theory and Observation
To appear in Ann. Rev. Astron. Astrophys. 2004 Neutron Star Cooling D. G. Yakovlev 1 and C. J. Pethick 2 1Ioffe Physical Technical Institute, Politekhnicheskaya 26, 194021 St.-Petersburg, Russia, e-mail: [email protected]ffe.ru 2NORDITA, The Nordic Institute for Theoretical Physics, Blegdamsvej 17, DK– 2100 Copenhagen Ø, Denmark, e-mail: [email protected] Abstract Observation of cooling neutron stars can potentially provide information about the states of matter at supernuclear densities. We review physical properties important for cooling such as neutrino emission processes and superfluidity in the stellar interior, surface envelopes of light elements due to accretion of matter and strong surface magnetic fields. The neutrino pro- cesses include the modified Urca process, and the direct Urca process for nucleons and exotic states of matter such as a pion condensate, kaon con- densate, or quark matter. The dependence of theoretical cooling curves on physical input and observations of thermal radiation from isolated neutron stars are described. The comparison of observation and theory leads to a unified interpretation in terms of three characteristic types of neutron stars: high-mass stars which cool primarily by some version of the direct Urca process; low-mass stars, which cool via slower processes; and medium-mass stars, which have an intermediate behavior. The related problem of thermal states of transiently accreting neutron stars with deep crustal burning of accreted matter is discussed in connection with observations of soft X-ray transients. arXiv:astro-ph/0402143v1 6 Feb 2004 1 INTRODUCTION Neutron stars are the most compact stars in the Universe. They have masses M ∼ 1.4 M⊙ and radii R ∼ 10 km, and they contain matter at supernuclear densities in their cores. -

Cooling Electrons in Semiconductor Devices: a Model of Evaporative Emission
PHYSICAL REVIEW B 75, 035316 ͑2007͒ Cooling electrons in semiconductor devices: A model of evaporative emission Thushari Jayasekera, Kieran Mullen, and Michael A. Morrison* Homer L. Dodge Department of Physics and Astronomy, The University of Oklahoma, 440 West Brooks Street, Norman, Oklahoma 73019-0225, USA ͑Received 4 April 2006; revised manuscript received 2 November 2006; published 11 January 2007͒ We discuss the theory of cooling electrons in solid-state devices via “evaporative emission.” Our model is based on filtering electron subbands in a quantum-wire device. When incident electrons in a higher-energy subband scatter out of the initial electron distribution, the system equilibrates to a different chemical potential and temperature than those of the incident electron distribution. We show that this re-equilibration can cause considerable cooling of the system. We discuss how the device geometry affects the final electron temperatures, and consider factors relevant to possible experiments. We demonstrate that one can therefore induce substantial electron cooling due to quantum effects in a room-temperature device. The resulting cooled electron population could be used for photodetection of optical frequencies corresponding to thermal energies near room temperature. DOI: 10.1103/PhysRevB.75.035316 PACS number͑s͒: 73.50.Lw, 73.23.Ϫb I. INTRODUCTION achieve electron cooling. We improve upon this result by switching to a “plus-junction” design, which improves the As electronic devices become smaller, they leave the re- cooling characteristics of the device. In Sec. IV we discuss gime of classical physics and enter the realm of quantum applications and realistic parameters for a device to cool physics. -

Water Investigating the Mpemba Effect
Teacher Resource Water Investigating the Mpemba effect. Water Terrific Scientific Campaign Investigation: Water Hello! Welcome to the Water Investigation from the Terrific Scientific campaign! At BBC Terrific Scientific, we think it is vital to develop science learning in primary schools across the UK. By taking part in this activity, you will be developing your class’s scientific thinking and investigative skills. At Key Stage 2 (Second Level), children need to: Develop investigative skills. Understand when it is important to control variables. Predict, observe and record results. Draw conclusions (which may generate new questions). Understand the need to repeat activities. Record what they see and not what they want to see. We have incorporated these principles into this exciting activity. We’ve made it suitable for primary classrooms by using readily available equipment and suggesting opportunities for support and differentiation. The BBC deems this activity safe if following some basic precautions. It is your responsibility as a School to carry out your own risk assessment and we recommend you consider the risks and mitigations we have described in this activity pack, as well as any risks which may be relevant to your specific class environment. TEACHERS RESOURCE 2 As well as these key working scientifically principles, we have made sure there are links to the science curriculum for each nation, as well as cross curricular opportunities for further learning. We think these are just as important, as they help to explain the relevance of Science and how it links to the world around us. On our website you will find a supporting ‘How to’ film which shows teachers and teaching assistants how to set up and carry out the experiment. -

Exam3 Practice
Materials Science and Engineering Department MSE 360, Test #3 ID number _______________________________ Name: _____________________________________ No notes, books, or information stored in calculator memories may be used. Cheating will be punished severely. All of your work must be written on these pages and turned in. Constants, equations, and other data are given on the last page of the exam. _______________________________________________________________________________ Problem 1-18: 2 points each (Key:1C2B3B4B5A6D 7B8D9B10B11A12A 13A14A15A16B17A18A) 1. A regular solution will likely form an ordered atomic structure if: d.) Ω = 0 b.) Ω > 0 c.) Ω < 0 2. With increasing temperature and constant pressure, the Gibbs free energy of a single phase A) increase, B) decrease 3. During annealing, the grain #2 represented in the right figure will A) be stable B) grow C) shrink 4. The mechanism of diffusion for a dilute solute atom of small atomic radius compared to the host material would be best described by: a.) Substitutional diffusion b.) Interstitial diffusion c.) Vacancy diffusion d.) all of the above 5. When Lf/Tm > 4R, where the Lf is the latent heat of fusion, Tm is the melting temperature, the liquid/solid interface is A) Smooth, B) Rough 6. The inhomogeneous nucleation is easier than the homogeneous nucleation because the inhomogeneous nucleation A. Has a critical nucleus with smaller diameter B. Has a critical nucleus with smaller volume C. Has a critical nucleus with smaller critical Gibbs free energy D. Both B and C 7. For heterogeneous nucleation in the grain interior, grain boundaries, grain edges and grain corners, their critical energy barriers can be described as A. -

Does Hot Water Freeze Faster Than Cold? Or Why Mpemba's Ice Cream Is a Discrepant Event
Palmer, W. P. (1993). Does hot water freeze faster than cold? or why Mpemba's ice cream is a discrepant event, The Journal of the Science Teacher Association of the Northern Territory, Volume 12, pp. 42-50. ABSTRACT A discrepant event is a happening contrary to our current beliefs. Discrepant events are said to be useful in clarifying concepts. This is one of the interesting features of current theories of constructivism. The story of Mpemba’s ice cream is quite well known, but it is the educational aspects of the experiment that are of interest in this story. Mpemba was a Tanzanian school boy, who was passionate about knowing the reason for his unexpected observations. His teacher made fun of him rather than carrying out the experiment and eventually a university lecturer listened to him and carried out the experiments. The paper focuses on the educational aspects of this experiment. The positive message is that although discrepant events are contrary to our current beliefs, they are useful in enabling learners to reconstruct concepts that have been imperfectly understood. The experimental observation is that if approximately equal amounts of a hot and a cold liquid are placed adjacent to each other in a fridge, then the hot liquid freezes first. This is a prime example of what science educators call a discrepant event (cognitive dissonance or cognitive conflict). _____________________________________________________________ DOES HOT WATER FREEZE FASTER THAN COLD? OR WHY MPEMBA'S ICE CREAM IS A DISCREPANT EVENT Bill Palmer Northern Territory University INTRODUCTION Firstly I will relate three stories about a surprising experiment.