This File Was Created by Scanning the Printed Publication. Text Errors Identified by the Software Have Been Corrected
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UNIVERSITY of ALASKA President’S Report
UNIVERSITY of ALASKA President’s Report 2006-2007 “The Next Thousand Days” Letter from UA President Mark Hamilton The University of Alaska stands at the brink of tremendous opportunity. The next thousand days takes us roughly to our 50th anniversary of Alaska’s statehood, achieved in 1959. As we head toward this celebration, I ask this question: Have we done all that we can to make the 49th star on the American flag the best that it can be? We’ve made great progress, but we must do more. We’re a state blessed with an abundance of natural resources, from oil and gas to fish and minerals. Have we made the most of these resources, providing a maximum benefit to the Alaskans who live here? Are we preparing our young people to take the legacy jobs that will exist long after a new gas pipeline has been constructed? The next thousand days present an awesome opportunity to reflect on the past and ensure a bright future. At UA this past year we awarded a record 3,223 degrees and certificates – the most ever in a single year. Public confidence in our state university system has never been stronger. More high school graduates than ever are choosing to attend college, and they are choosing UA. The UA Scholars program has been wildly successful, beyond anything I had hoped for when it was first introduced in 1999. In short, we’re on a roll. We have many goals over the next thousand days. They roughly fall into one of these three categories: • Workforce Development – Strengthening programs aimed at producing an educated and trained workforce for Alaska, -

UAF Administration, Faculty and Emeriti
UAF Administration, Faculty and Emeriti UA BOARD OF REGENTS Dale Anderson ....................................................................................................................................2012-2021 Sheri Buretta ....................................................................................................................................... 2015-2023 John Davies ......................................................................................................................................... 2015-2023 Kenneth J. Fisher, Secretary ............................................................................................................. 2009-2017 Jyotsna Heckman, Chair ....................................................................................................................2011-2019 Mary K. Hughes ................................................................................................................................. 2002-2017 Stacey Lucason ....................................................................................................................................2015-2017 Gloria O’Neill, Treasurer ...................................................................................................................2013-2021 Lisa Parker .......................................................................................................................................... 2015-2023 Michael Powers, Vice Chair ..............................................................................................................2011-2019 -

Geography Program Expands to Integrate Statewide Instruction, Research, and Outreach
Natural Resource News, Vol. 05, No. 2 (August 2005) Item Type Journal Publisher UAF School of Natural Resources and Agricultural Sciences Download date 24/09/2021 12:31:29 Link to Item http://hdl.handle.net/11122/2793 UAF School of Natural Resources and Agricultural Sciences Volume 5, number 2 August 2005 Geography program expands to integrate statewide instruction, research, and outreach “Locally, regionally, and at A new Alaska Integrated Geography Program based at SNRAS joint research proposals, and a global planetary level, life will create a University of Alaska framework for cooperative allow UA to take advantage of modifies Earth environments programs throughout the UA system. Based at SNRAS on our arctic and polar expertise.” as much as environments shape life. Few changes have the UAF campus, the innovative program will involve people K-12 teacher education and so touched vista, ecosystem from all UA campuses in geography-related research, instruc- curriculum development will and global cycling as has the tion, and outreach. The state’s only baccalaureate degrees in also be a key component of the evolution of the angiosperms geography are offered at UAF. new geography program. Sfraga (plants with flowers). In the “This development fits in with the vision of UA president has already established strong lush Cretaceous forests of fern Mark Hamilton and the Board of Regents, who have encour- ties with the Alaska Geographic and cycad, new colors and aged coordination of university-wide programs that take ad- Alliance and sees a strong pres- scents arise....” —A Walk vantage of UA expertise and our strategic location,” said Dean ence for the AGA within the Through Time (Page 2) Carol Lewis. -

UAA PERFORMANCE ‘18 October 2018
University of Alaska Anchorage UAA PERFORMANCE ‘18 October 2018 Produced by OFFICE OF INSTITUTIONAL EFFECTIVENESS, ENGAGEMENT AND ACADEMIC SUPPORT Renee Carter-Chapman, Senior Vice Provost John Dede, Research Analyst Sara Juday, Writer/Editor/Graphic Designer Ester Bayne, Editorial Assistant In partnership with OFFICE OF INSTITUTIONAL RESEARCH Erin Holmes, Associate Vice Provost Yuan-Fang Dong, Senior Research Associate Ginger Mongeau, Research Associate Coy Gullett, Senior Research Associate OFFICE OF BUDGET Kelly Thorngren, Director OFFICE OF RESEARCH & GRADUATE STUDIES Helena Wisniewski, Vice Provost George Kamberov, Interim Vice Provost for Research Christie Trueblood, Director of Finance Photos provided by James Evans, Philip Hall, Ted Kincaid, Joshua Borough, John DeLapp, Skip Hickey, UAA Athletics Cover design by Joe Nedland, Senior Graphic Artist, University Relations And special thanks to Academic Affairs Business Enterprise Institute Chancellor’s Office Dean of Students Office of Development Environmental Health & Safety/Risk Management University Advancement NOTICE OF NONDISCRIMINATION: The University of Alaska is an affirmative action/equal opportunity employer and educational institution. The University of Alaska does not discriminate on the basis of race, religion, color, national origin, citizenship, age, sex, physical or mental disability, status as a protected veteran, marital status, changes in marital status, pregnancy, childbirth or related medical conditions, parenthood, sexual orientation, gender identity, political affiliation or belief, genetic information, or other legally protected status. The University’s commitment to nondiscrimination, including against sex discrimination, applies to students, employees, and applicants for admission and employment. Contact information, applicable laws, and complaint procedures are included on UA’s statement of nondiscrimination available at www.alaska.edu/nondiscrimination. FRONT COVER: Center photo: Master of Engineering Science graduates Stefanie Armstrong, Michael Ulroan, and Forest Rose Walker. -

FY03 Redbook
Fiscal Year 2003 Operating & Capital Budget Requests Fiscal Year 2003 Operating & Capital Budget Requests Prepared by Statewide Budget & Institutional Research PO BOX 755260 Fairbanks, AK 99775-5260 (907) 474-5889 Fax (907) 474-6682 www.alaska.edu/oir with assistance from: Statewide Office of Public Affairs Website References FY03 Operating & Capital Budget Request www.alaska.edu/swbudget/redindex.htm This publication can be found at this link. University of Alaska www.alaska.edu/ Systemwide homepage University of Alaska, Statewide Budget & Institutional Research www.alaska.edu/oir/ Links to information about the University of Alaska budget and institutional research topics. University of Alaska, Statewide Budget Development www.alaska.edu/swbudget/ Links to reports on budgets and budget development unit level reporting, deferred maintenance and other miscella- neous reports. University of Alaska, Academic Affairs www.alaska.edu/swacad Links to initiative information and forms. University of Alaska, UA in Review www.alaska.edu/oir/Review/index.html University of Alaska factbooks by year. Includes information about budgets, students, academics, faculty and staff, and institutional data. University of Alaska, Statewide University Relations www.alaska.edu/state Links to legislation and budget information with the potential to impact the University of Alaska. University of Alaska, Board of Regents www.alaska.edu/bor/ Links to information about the Board of Regents’ activities and policies. Alaska Legislature, Legislative Finance Division www.legfin.state.ak.us/ Information and links to state budget data. State of Alaska, Office of Management and Budget www.gov.state.ak.us/omb/akomb.htm Links to OMB state budget information. State of Alaska, University of Alaska Operating Budget Detail www.gov.state.ak.us/omb/03OMB/budget/UA/UA_Cover.html This is an OMB site that contains the University's submission for Capital and Operating Budget. -
7. President's Report 2008-09
Preparing Alaska for the Next 50 Years University of Alaska President’s Report 2008-2009 Preparing Alaska for the Next 50 Years University of Alaska President’s Report 2008-2009 A letter from UA President Mark Hamilton This past year has been one of tremendous change and challenge. We’ve seen oil prices skyrocket and then plunge, our financial markets spiral downward, and our friends, family and colleagues, mostly in the Lower 48, suffer the loss of a home, job or a loved one in war. We’ve watched our governor launch into the national and world spotlight as a vice presidential nominee, and we witnessed the first African American elected president of our country. We also somberly said good-bye to our longest-running public servant, U.S. Senator Ted Stevens, who lost re-election after 40 years. This is Alaska’s 50th anniversary of statehood. The years since 1959 haven’t been perfect—we often faced extreme challenges in the past, and we will again in the future. The University of Alaska is on solid footing while we adapt to fiscal realities and continue to serve state needs. Tough times can cause people to do one of two things; wring their hands in despair… or raise them up. At the University of Alaska, we’re raising up our hands to say, yes, we can meet the challenges of the next 50 years. We’ll work on Alaska’s tough issues, including educating the next generation, providing a trained and qualified workforce for jobs in Alaska, and ensuring our young people have the best opportunities possible, from pre- school through postsecondary education. -

Original Contains J
APPLICATIONS OF REMOTE-SENSING DATA IN ALASKA A Cooperative Program of the University of Alaska with User Organizations, Including Local, State and Federal Government Agencies ORIGINAL CONTAINS J. M. Miller SOLOR ILLUSTRATIogS Geophysical Institute University of Alaska Fairbanks, Alaska 99701 I(NASA-CR-156996) APPLICATIONS OF REMOTE N78-23533 1 SENSING DATA IN ALASKA: A COOPERATIVE' PROGRAM 01-TUE UNIVERSITY OF ALASKA WITH USER ORGANIZATIONS, INCLUDING LOCAL, STATE Unclas AND- FEDERAL GOVERNMENT (Alaska Univ., G3/43 17723 ANNUAL REPORT Grant NGL 02-001-092 Period Covered July 1, 1975 - June 30, 1976 Prepared for: National Aeronautics and Space Administration Office of University Affairs Washington, DC 20546 TABLE OF CONTENTS INTRODUCTION I SUMMARY OF ACTIVITIES 5 Coordination and Information Exchange Data Library 9 Data Processing Services 14 Training & Workshops 20 -Alaskan Needs for Resource Information 23 Cooperative Projects 25 USD1/Bureau of Indian Affairs 26 Applications of Thermal-Infrared Data 36 Alaska Department of Environmental 'Conservation 39 USDA/Soil Conservation Service-Range Inventory 44 Chugach'Native Corporation 45 BP-Alaska Inc. 46 North Slope Borough 47 USDA/Soil Conservation Service-Flooding 50 Corps of Engineers 52 National Oceanic and Atmospheric Administration 53 Alaska State Troopers & U.S. Secret Service 56 Conferences and.Meetings Attended 60 CONCLUSIONS & RECOMMENDATIONS 62 APPENDICES A - List of Publications B - Catalog of Alaskan Landsat Data C - Horizons on Display D - USDA Soil Conservation Service Reports E - Alaska Rural Development Council i INTRODUCTION Even more so than the private sector, agencies of federal, state and regional governments have an increasing need for detailed information in areas related to natural resources for policy formulation, program development and evaluation of results. -

Guide to Alaska Geologic and Mineral Information 2004 INFORMATION CIRCULAR 44 Division of Geological & Geophysical Surveys
Guide to Alaska Geologic and Mineral Information 2004 INFORMATION CIRCULAR 44 Division of Geological & Geophysical Surveys in cooperation with: United States Geological Survey • University of Alaska Fairbanks Rasmuson Library • Geophysical Institute Keith Mather Library • University of Alaska Anchorage Consortium Library • Bureau of Land Management Juneau - John Rishel Mineral Information Center • ARLIS (Alaska Resources Library and Information Services) • Alaska State Library Guide to Alaska Geologic and Mineral Information 2004 E. Ellen Daley Ph.D., Editor Information Circular 44 Alaska Division of Geological & Geophysical Surveys in cooperation with • United States Geological Survey • University of Alaska Anchorage Consortium Library • Bureau of Land Management Juneau - John Rishel Mineral • University of Alaska Fairbanks Information Center Rasmuson Library • ARLIS (Alaska Resources Library • University of Alaska Fairbanks and Information Services) Geophysical Institute Keith Mather Library • Alaska State Library i Foreword Until his death in 1983, Edward H. Cobb of the U.S. Geological Survey main- tained a truly exceptional database on the geology and mineral deposits of Alaska—manifested in hundreds of published mineral locality maps, bibliogra- phies, and compilations of Alaska references. His work anticipated a fundamen- tal need to define the mineral endowment of Alaska during the long process that culminated in the Alaska National Interest Lands Conservation Act of 1981 (ANILCA). Almost 20 years later, Ed Cobb’s maps, bibliographies, and publica- tions, outdated as they have become in some ways, remain a mainstay of Alaska geology, land planning, and mineral exploration. Geology is a cumulative science built on the work of our predecessors. No one produced a better regional geologic information database for others to build on than did Ed Cobb in Alaska. -

Bob Mccoy Director, Geophysical Institute University of Alaska Fairbanks
Arctic Space Weather Research in Alaska 11 Bob McCoy Director, Geophysical Institute www.gi.Alaska.edu University of Alaska Fairbanks [email protected] Major State-owned or University-owned Suborbital . Ionospheric Research Facilities PFRR Fairbanks HAARP PSC-A Suborbital & Orbital • Poker Flat Research Range (PFRR) • High-frequency Active Auroral Research Program (HAARP) • Pacific Spaceport Complex Alaska (PSCA) 2 From Mark Conde Poker Flat Research Range Largest land-based rocket range Only rocket range owned by a university 35 miles from campus PF Incoherent Scatter Radar A regional, high-latitude ionospheric laboratory Cameras PFISR SuperDARN Rockets GNSS current status, green already installed, blue upcoming, gray are potential sites Don Hampton Anthea Coster Alaska Earthquake Center I,D • Report ~ 50,000 earthquakes/yr • 50 earthquakes > M5; 6 > M6; M7 (every other year) • Publishes Tsunami hazard maps for Alaska EarthScope in Alaska & Western Canada is complete – 193 stations Missile Defense Agency (MDA) Long Range Discrimination Radar LRDR Clear Air Force Station High-frequency Active Aurora Research Program (HAARP) Reaching Out & Touching the Ionosphere • Gakona AK - 62.39 deg, 145.15 deg (West) • 33 acre phased HF transmitter array; • 2.8 to 10 MHz; • Multiple beams & transmission to 30 elevation angle • 5 x 3600 hp diesel engines; 3.6 MW; • $290M (half Congressional earmarks + half AFRL, ONR & DARPA) HF Ionospheric Heating ULF, ELF, VLF wave injection Heating Duct creation Airglow Rotating Wave generation beams Currents Chemistry -

Finsys1:/Fixed Assets/Contacts/Perdir.Doc 02/10/2017
PROPERTY PERSONNEL DIRECTORY UNIVERSITY OF ALASKA ANCHORAGE UAA General Support Services, 3211 Providence Dr., bookstore basement, Anchorage, AK 99508 LynnAnn Briske, property Coordinator, [email protected] .................................................................... 786-1195 Kimberley Stanford, Director, [email protected] ............................................................................. 786-4668 UAA Kenai Peninsula College, 156 College Drive, Soldotna, AK 99669 .................................................. FAX - 262-0322 Lake Nash, Accounts Payable Travel Technician, [email protected] ..................................................... 262-0308 UAA Kodiak College, 117 Benny Benson Dr., Kodiak, AK 99615 ............................................................ FAX - 486-1240 Grace Bachini, Property Coordinator, [email protected] .................................................................. 486-1216 Peggy Rauwolf, Property Manager, [email protected] ...................................................................... 486-1217 UAA Matanuska-Susitna College, 8295 East College Drive, Palmer, AK 99645 ...................................... FAX - 745-9711 Susan Elwell, Property Coordinator, [email protected] ...................................................................... 745-9724 UAA Prince William Sound Community College, PO Box 97, 303 Lowe St, Valdez, AK 99686-0097 ...... FAX - 835-2593 Nan Haley, Property Coordinator, [email protected] ......................................................................... -

Sun Star Vol 34 No 13 ( November 25Th, 2014)
The Sun Star Vol 34 No 13 ( November 25th, 2014) Publisher The Sun Star Download date 09/10/2021 04:42:27 Link to Item http://hdl.handle.net/11122/7054 The VolumeSUN XXXIV, Issue # 13 STAR Tuesday November 25, 2014 UAF works with Dartmouth in Arctic initiative pg. 7 Adjunct Faculty discuss payment and working conditions by Travis Olsen the lack of employment tracts common for full- ment," according to a search (PAIR) data last gineering student Kyle Sun Star consistency and not hav- time faculty, are required letter submitted in June updated in fall of 2013, Milne, he said, "That's ing offices to meet with to hold office hours for to the University of Alas- UAF had employed 331 way more than I thought, A handful of adjunct students in private. students, as well as attend ka by Pete Ford, regional adjunct faculty that se- I had no idea it was that faculty members met last Adjunct faculty mem- any university required manager of the adjuncts' mester, compared to the many." month with union repre- bers, who teach 35per- training, such as the re- union. Adjunct facul- listed 375 full-time in- Most adjunct faculty sentatives to discuss how cent of credits offered at cent Title IX training. ty are still having issues structional faculty. Many members are paid a flat to address their lack of UAF according to uni- The recently man- getting compensated for students are unaware of rate to teach a course, compensation for train- versity data, are hired dated Title IX training their time. -
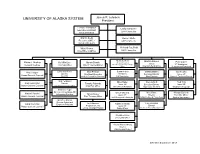
UNIVERSITY of ALASKA SYSTEM James R
UNIVERSITY OF ALASKA SYSTEM James R. Johnsen President Teresa Thompson Cathy Sandeen Executive Assistant UAA Chancellor to the President Brandi Berg Daniel White Executive Officer UAF Chancellor Board of Regents Mary Gower Richard Caulfield Chief Title IX Officer UAS Chancellor Michelle Rizk Martha Mason Michael Hostina Keli McGee Myron Dosch VP Univ Relations & Paul Layer Interim General Counsel Chief HR Officer Chief Finance Officer Chief Strategy, Planning & Budget VP Academics, Officer Chief Info Tech Officer Students and Research Erika Van Flein Susan Foley Matt Cooper Nikki Pittman Martha Mason Saichi Oba Director Chief Audit Executive President, Executive Director Assoc VP Assoc General Counsel Benefits Audit & Consulting Services UA Foundation User Services Student & Enrollment Strategy Tara Ferguson Miles Baker Rory O’Neill Fred Villa Andy Harrington Director Timothy Edwards Assoc VP Executive Director Assoc VP Assoc General Counsel Classification/Compensation Chief Risk Officer Government Relations Application Services Workforce Programs Michelle Pope Director, Accounting & HR Rachel Plumlee Gwen Gruenig Mike Brase Morgan Dufseth Information Systems Tammi Weaver Assoc VP Director Executive Officer Assoc General Counsel Chief Treasury Officer Inst’l Research & Analysis Infrastructure & Cloud Svcs System Governance Geoffrey Bacon Director, Labor and Lael Oldmixon Tom Langdon Dana Merriman Employee Relations Robbie Graham Director Assoc General Counsel Program Director Assoc VP College Savings Program Public Affairs Service & Project