Neutron Interactions with Matter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neutron Stars
Chandra X-Ray Observatory X-Ray Astronomy Field Guide Neutron Stars Ordinary matter, or the stuff we and everything around us is made of, consists largely of empty space. Even a rock is mostly empty space. This is because matter is made of atoms. An atom is a cloud of electrons orbiting around a nucleus composed of protons and neutrons. The nucleus contains more than 99.9 percent of the mass of an atom, yet it has a diameter of only 1/100,000 that of the electron cloud. The electrons themselves take up little space, but the pattern of their orbit defines the size of the atom, which is therefore 99.9999999999999% Chandra Image of Vela Pulsar open space! (NASA/PSU/G.Pavlov et al. What we perceive as painfully solid when we bump against a rock is really a hurly-burly of electrons moving through empty space so fast that we can't see—or feel—the emptiness. What would matter look like if it weren't empty, if we could crush the electron cloud down to the size of the nucleus? Suppose we could generate a force strong enough to crush all the emptiness out of a rock roughly the size of a football stadium. The rock would be squeezed down to the size of a grain of sand and would still weigh 4 million tons! Such extreme forces occur in nature when the central part of a massive star collapses to form a neutron star. The atoms are crushed completely, and the electrons are jammed inside the protons to form a star composed almost entirely of neutrons. -

The Five Common Particles
The Five Common Particles The world around you consists of only three particles: protons, neutrons, and electrons. Protons and neutrons form the nuclei of atoms, and electrons glue everything together and create chemicals and materials. Along with the photon and the neutrino, these particles are essentially the only ones that exist in our solar system, because all the other subatomic particles have half-lives of typically 10-9 second or less, and vanish almost the instant they are created by nuclear reactions in the Sun, etc. Particles interact via the four fundamental forces of nature. Some basic properties of these forces are summarized below. (Other aspects of the fundamental forces are also discussed in the Summary of Particle Physics document on this web site.) Force Range Common Particles It Affects Conserved Quantity gravity infinite neutron, proton, electron, neutrino, photon mass-energy electromagnetic infinite proton, electron, photon charge -14 strong nuclear force ≈ 10 m neutron, proton baryon number -15 weak nuclear force ≈ 10 m neutron, proton, electron, neutrino lepton number Every particle in nature has specific values of all four of the conserved quantities associated with each force. The values for the five common particles are: Particle Rest Mass1 Charge2 Baryon # Lepton # proton 938.3 MeV/c2 +1 e +1 0 neutron 939.6 MeV/c2 0 +1 0 electron 0.511 MeV/c2 -1 e 0 +1 neutrino ≈ 1 eV/c2 0 0 +1 photon 0 eV/c2 0 0 0 1) MeV = mega-electron-volt = 106 eV. It is customary in particle physics to measure the mass of a particle in terms of how much energy it would represent if it were converted via E = mc2. -

Lecture 6: Spectroscopy and Photochemistry II
Lecture 6: Spectroscopy and Photochemistry II Required Reading: FP Chapter 3 Suggested Reading: SP Chapter 3 Atmospheric Chemistry CHEM-5151 / ATOC-5151 Spring 2005 Prof. Jose-Luis Jimenez Outline of Lecture • The Sun as a radiation source • Attenuation from the atmosphere – Scattering by gases & aerosols – Absorption by gases • Beer-Lamber law • Atmospheric photochemistry – Calculation of photolysis rates – Radiation fluxes – Radiation models 1 Reminder of EM Spectrum Blackbody Radiation Linear Scale Log Scale From R.P. Turco, Earth Under Siege: From Air Pollution to Global Change, Oxford UP, 2002. 2 Solar & Earth Radiation Spectra • Sun is a radiation source with an effective blackbody temperature of about 5800 K • Earth receives circa 1368 W/m2 of energy from solar radiation From Turco From S. Nidkorodov • Question: are relative vertical scales ok in right plot? Solar Radiation Spectrum II From F-P&P •Solar spectrum is strongly modulated by atmospheric scattering and absorption From Turco 3 Solar Radiation Spectrum III UV Photon Energy ↑ C B A From Turco Solar Radiation Spectrum IV • Solar spectrum is strongly O3 modulated by atmospheric absorptions O 2 • Remember that UV photons have most energy –O2 absorbs extreme UV in mesosphere; O3 absorbs most UV in stratosphere – Chemistry of those regions partially driven by those absorptions – Only light with λ>290 nm penetrates into the lower troposphere – Biomolecules have same bonds (e.g. C-H), bonds can break with UV absorption => damage to life • Importance of protection From F-P&P provided by O3 layer 4 Solar Radiation Spectrum vs. altitude From F-P&P • Very high energy photons are depleted high up in the atmosphere • Some photochemistry is possible in stratosphere but not in troposphere • Only λ > 290 nm in trop. -
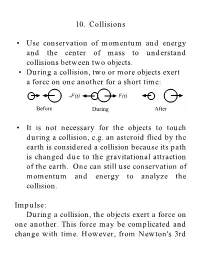
10. Collisions • Use Conservation of Momentum and Energy and The
10. Collisions • Use conservation of momentum and energy and the center of mass to understand collisions between two objects. • During a collision, two or more objects exert a force on one another for a short time: -F(t) F(t) Before During After • It is not necessary for the objects to touch during a collision, e.g. an asteroid flied by the earth is considered a collision because its path is changed due to the gravitational attraction of the earth. One can still use conservation of momentum and energy to analyze the collision. Impulse: During a collision, the objects exert a force on one another. This force may be complicated and change with time. However, from Newton's 3rd Law, the two objects must exert an equal and opposite force on one another. F(t) t ti tf Dt From Newton'sr 2nd Law: dp r = F (t) dt r r dp = F (t)dt r r r r tf p f - pi = Dp = ò F (t)dt ti The change in the momentum is defined as the impulse of the collision. • Impulse is a vector quantity. Impulse-Linear Momentum Theorem: In a collision, the impulse on an object is equal to the change in momentum: r r J = Dp Conservation of Linear Momentum: In a system of two or more particles that are colliding, the forces that these objects exert on one another are internal forces. These internal forces cannot change the momentum of the system. Only an external force can change the momentum. The linear momentum of a closed isolated system is conserved during a collision of objects within the system. -

Photons from 252Cf and 241Am-Be Neutron Sources H
Neutronenbestrahlungsraum Calibration Laboratory LB6411 Am-Be Quelle [email protected] Photons from 252Cf and 241Am-Be neutron sources H. Hoedlmoser, M. Boschung, K. Meier and S. Mayer Paul Scherrer Institut, CH-5232 Villigen PSI, Switzerland At the accredited PSI calibration laboratory neutron reference fields traceable to the standards of the Physikalisch-Technische Bundesanstalt (PTB) in Germany are available for the calibration of ambient and personal dose equivalent (rate) meters and passive dosimeters. The photon contribution to H*(10) in the neutron fields of the 252Cf and 241Am-Be sources was measured using various photon dose rate meters and active and passive dosimeters. Measuring photons from a neutron source involves considerable uncertainties due to the presence of neutrons, due to a non-zero neutron sensitivity of the photon detector and due to the energy response of the photon detectors. Therefore eight independent detectors and methods were used to obtain a reliable estimate for the photon contribution as an average of the individual methods. For the 241Am-Be source a photon contribution of approximately 4.9% was determined and for the 252Cf source a contribution of 3.6%. 1) Photon detectors 2) Photons from 252Cf and 241Am-Be neutron sources Figure 1 252Cf decays through -emission (~97%) and through spontaneous fission (~3%) with a half-life of 2.65 y. Both processes are accompanied by photon radiation. Furthermore the spectrum of fission products contains radioactive elements that produce additional gamma photons. In the 241Am-Be source 241Am decays through -emission and various low energy emissions: 241Am237Np + + with a half life of 432.6 y. -

1 Standard Model: Successes and Problems
Searching for new particles at the Large Hadron Collider James Hirschauer (Fermi National Accelerator Laboratory) Sambamurti Memorial Lecture : August 7, 2017 Our current theory of the most fundamental laws of physics, known as the standard model (SM), works very well to explain many aspects of nature. Most recently, the Higgs boson, predicted to exist in the late 1960s, was discovered by the CMS and ATLAS collaborations at the Large Hadron Collider at CERN in 2012 [1] marking the first observation of the full spectrum of predicted SM particles. Despite the great success of this theory, there are several aspects of nature for which the SM description is completely lacking or unsatisfactory, including the identity of the astronomically observed dark matter and the mass of newly discovered Higgs boson. These and other apparent limitations of the SM motivate the search for new phenomena beyond the SM either directly at the LHC or indirectly with lower energy, high precision experiments. In these proceedings, the successes and some of the shortcomings of the SM are described, followed by a description of the methods and status of the search for new phenomena at the LHC, with some focus on supersymmetry (SUSY) [2], a specific theory of physics beyond the standard model (BSM). 1 Standard model: successes and problems The standard model of particle physics describes the interactions of fundamental matter particles (quarks and leptons) via the fundamental forces (mediated by the force carrying particles: the photon, gluon, and weak bosons). The Higgs boson, also a fundamental SM particle, plays a central role in the mechanism that determines the masses of the photon and weak bosons, as well as the rest of the standard model particles. -

Part B. Radiation Sources Radiation Safety
Part B. Radiation sources Radiation Safety 1. Interaction of electrons-e with the matter −31 me = 9.11×10 kg ; E = m ec2 = 0.511 MeV; qe = -e 2. Interaction of photons-γ with the matter mγ = 0 kg ; E γ = 0 eV; q γ = 0 3. Interaction of neutrons-n with the matter −27 mn = 1.68 × 10 kg ; En = 939.57 MeV; qn = 0 4. Interaction of protons-p with the matter −27 mp = 1.67 × 10 kg ; Ep = 938.27 MeV; qp = +e Note: for any nucleus A: mass number – nucleons number A = Z + N Z: atomic number – proton (charge) number N: neutron number 1 / 35 1. Interaction of electrons with the matter Radiation Safety The physical processes: 1. Ionization losses inelastic collisions with orbital electrons 2. Bremsstrahlung losses inelastic collisions with atomic nuclei 3. Rutherford scattering elastic collisions with atomic nuclei Positrons at nearly rest energy: annihilation emission of two 511 keV photons 2 / 35 1. Interaction of electrons with1.E+02 the matter Radiation Safety ) 1.E+03 -1 Graphite – Z = 6 .g 2 1.E+01 ) Lead – Z = 82 -1 .g 2 1.E+02 1.E+00 1.E+01 1.E-01 1.E+00 collision 1.E-02 radiative collision 1.E-01 stopping pow er (MeV.cm total radiative 1.E-03 stopping pow er (MeV.cm total 1.E-02 1.E-01 1.E+00 1.E+01 1.E+02 1.E+03 1.E-02 energy (MeV) 1.E-02 1.E-01 1.E+00 1.E+01 1.E+02 1.E+03 Electrons – stopping power 1.E+02 energy (MeV) S 1 dE ) === -1 Copper – Z = 29 ρρρ ρρρ dl .g 2 1.E+01 S 1 dE 1 dE === +++ ρρρ ρρρ dl coll ρρρ dl rad 1 dE 1.E+00 : mass stopping power (MeV.cm 2 g. -

7. Gamma and X-Ray Interactions in Matter
Photon interactions in matter Gamma- and X-Ray • Compton effect • Photoelectric effect Interactions in Matter • Pair production • Rayleigh (coherent) scattering Chapter 7 • Photonuclear interactions F.A. Attix, Introduction to Radiological Kinematics Physics and Radiation Dosimetry Interaction cross sections Energy-transfer cross sections Mass attenuation coefficients 1 2 Compton interaction A.H. Compton • Inelastic photon scattering by an electron • Arthur Holly Compton (September 10, 1892 – March 15, 1962) • Main assumption: the electron struck by the • Received Nobel prize in physics 1927 for incoming photon is unbound and stationary his discovery of the Compton effect – The largest contribution from binding is under • Was a key figure in the Manhattan Project, condition of high Z, low energy and creation of first nuclear reactor, which went critical in December 1942 – Under these conditions photoelectric effect is dominant Born and buried in • Consider two aspects: kinematics and cross Wooster, OH http://en.wikipedia.org/wiki/Arthur_Compton sections http://www.findagrave.com/cgi-bin/fg.cgi?page=gr&GRid=22551 3 4 Compton interaction: Kinematics Compton interaction: Kinematics • An earlier theory of -ray scattering by Thomson, based on observations only at low energies, predicted that the scattered photon should always have the same energy as the incident one, regardless of h or • The failure of the Thomson theory to describe high-energy photon scattering necessitated the • Inelastic collision • After the collision the electron departs -

12 Scattering in Three Dimensions
12 Scattering in three dimensions 12.1 Cross sections and geometry Most experiments in physics consist of sending one particle to collide with another, and looking at what comes out. The quantity we can usually measure is the scattering cross section: by analogy with classical scattering of hard spheres, we assuming that scattering occurs if the particles ‘hit’ each other. The cross section is the apparent ‘target area’. The total scattering cross section can be determined by the reduction in intensity of a beam of particles passing through a region on ‘targets’, while the differential scattering cross section requires detecting the scattered particles at different angles. We will use spherical polar coordinates, with the scattering potential located at the origin and the plane wave incident flux parallel to the z direction. In this coordinate system, scattering processes dσ are symmetric about φ, so dΩ will be independent of φ. We will also use a purely classical concept, the impact parameter b which is defined as the distance of the incident particle from the z-axis prior to scattering. S(k) δΩ I(k) θ z φ Figure 11: Standard spherical coordinate geometry for scattering 12.2 The Born Approximation We can use time-dependent perturbation theory to do an approximate calculation of the cross- section. Provided that the interaction between particle and scattering centre is localised to the region around r = 0, we can regard the incident and scattered particles as free when they are far from the scattering centre. We just need the result that we obtained for a constant perturbation, Fermi’s Golden Rule, to compute the rate of transitions between the initial state (free particle of momentum p) to the final state (free particle of momentum p0). -

Photon Cross Sections, Attenuation Coefficients, and Energy Absorption Coefficients from 10 Kev to 100 Gev*
1 of Stanaaros National Bureau Mmin. Bids- r'' Library. Ml gEP 2 5 1969 NSRDS-NBS 29 . A111D1 ^67174 tioton Cross Sections, i NBS Attenuation Coefficients, and & TECH RTC. 1 NATL INST OF STANDARDS _nergy Absorption Coefficients From 10 keV to 100 GeV U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS T X J ". j NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation’s central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To this end the Bureau conducts research and provides central national services in four broad program areas. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Radiation Research, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scientific community, industry, and com- merce. The Institute consists of an Office of Measurement Services and the following technical divisions: Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic and Molec- ular Physics—Radio Physics -—Radio Engineering -—Time and Frequency -—Astro- physics -—Cryogenics. -

12. Elastic Collisions A) Overview B) Elastic Collisions V
12. Elastic Collisions A) Overview In this unit, our focus will be on elastic collisions, namely those collisions in which the only forces that act during the collision are conservative forces. In these collisions, the sum of the kinetic energies of the objects is conserved. We will find that the description of these collisions is significantly simplified in the center of mass frame of the colliding objects. In particular, we will discover that, in this frame, the speed of each object after the collision is the same as its speed before the collision. B) Elastic Collisions In the last unit, we discussed the important topic of momentum conservation. In particular, we found that when the sum of the external forces acting on a system of particles is zero, then the total momentum of the system, defined as the vector sum of the individual momenta, will be conserved. We also determined that the kinetic energy of the system, defined to be the sum of the individual kinetic energies, is not necessarily conserved in collisions. Whether or not this energy is conserved is determined by the details of the forces that the components of the system exert on each other. In the last unit, our focus was on inelastic collisions, those collisions in which the kinetic energy of the system was not conserved. In particular non-conservative work was done by the forces that the individual objects exerted on each other during the collision. In this unit, we will look at examples in which the only forces that act during the collision are conservative forces. -

Glossary of Scientific Terms in the Mystery of Matter
GLOSSARY OF SCIENTIFIC TERMS IN THE MYSTERY OF MATTER Term Definition Section acid A substance that has a pH of less than 7 and that can react with 1 metals and other substances. air The mixture of oxygen, nitrogen, and other gasses that is consistently 1 present around us. alchemist A person who practices a form of chemistry from the Middle Ages 1 that was concerned with transforming various metals into gold. Alchemy A type of science and philosophy from the Middle Ages that 1 attempted to perform unusual experiments, taking something ordinary and turning it into something extraordinary. alkali metals Any of a group of soft metallic elements that form alkali solutions 3 when they combine with water. They include lithium, sodium, potassium, rubidium, cesium, and francium. alkaline earth Any of a group of metallic elements that includes beryllium, 3 metals magnesium, calcium, strontium, barium, and radium. alpha particle A positively charged particle, indistinguishable from a helium atom 5, 6 nucleus and consisting of two protons and two neutrons. alpha decay A type of radioactive decay in which a nucleus emits 6 an alpha particle. aplastic anemia A disorder of the bone marrow that results in too few blood cells. 4 apothecary The person in a pharmacy who distributes medicine. 1 atom The smallest component of an element that shares the chemical 1, 2, 3, 4, 5, 6 properties of the element and contains a nucleus with neutrons, protons, and electrons. atomic bomb A bomb whose explosive force comes from a chain reaction based on 6 nuclear fission. atomic number The number of protons in the nucleus of an atom.