Journal of Earth and Environmental Sciences Panaskar DB, Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ifji=D@Dk;Z'kkgk@Ppkzl=@Ifj”Kn
ACADEMIC PLANNING & DEVELOPMENT SECTION Phone : (02462) 229242 Website: srtmun.ac.in Fax : (02462) 229574 E-mail: [email protected] ifji=d@dk;Z’kkGk@ppkZl=@ifj”kn@ifjlaokn 2019&20 ;k ifji=dkUo;s loZ lacf/krkl dGfo.;kr ;srs dh] fnxacjjko fcanw dyk] okf.kT; vkf.k foKku egkfo|ky;] Hkksdj ft- ukansM ;kaP;k orhus Intellectual Property Rights (IPR) & Patent Filing ¼fn-26 tqyS 2019½ vkf.k Research Methodology ¼fn- 27 tqyS 2019½ ;k fo”k;akP;k vH;kldzekoj vk/kkjhr fo|kihBLrjh; dk;Z’kkGsps vk;kstu dj.;kr vkysys vkgs- lnjhy dk;Z’kkGsl izLrqr fo|kihBk’kh layfXur loZ egkfo|ky;krhy lacf/kr fo”k;kaP;k lgHkkxh gks.kk&;k izk/;kidkauk R;k&R;k egkfo|ky;kP;korhus drZO; jtk (Duty Leave) eatqj dj.;kr ;koh- Lok@& ßKkurhFkZÞ ifjlj fo”.kqiwjh] ukansM midqylfpo Tkk-dz- ‘kSfufofo@ifji=d@dk;Z’kkGk@2019&20@508 ‘kS{kf.kd fu;kstu o fodkl foHkkx fn- 13@07@2019 izr % 1- ek-izkpk;Z] fnxacjjko fcanw dyk] okf.kT; vkf.k foKku egkfo|ky;] Hkksdj ft- ukansM ;kauk ;k ifji=dkph izr nsÅu dGfo.;kr ;srs dh] mDr dk;Zdzekr lgHkkxh gks.kk&;k lacf/krkauk vkiY;k Lrjko:u Lora=i.ks dGfo.;kr ;kos- gh fouarh- 2- flLVhe ,DliVZ] izLrqr fo|kihB ;kauk izr nsÅu dGfo.;kr ;srs dh] lnjhy ifji=d fo|kihBkP;k ladsrLFkGkoj izdkf’kr djkos- Patrons President, Late. Digambarrao Bindu Smarak Samiti’s, Bhokar, MS, India Ex, Minister, Govt. of Maharashtra, India 27th July, 2019. 27th July, 2019. -

Reg. No Name in Full Residential Address Gender Contact No. Email Id Remarks 9421864344 022 25401313 / 9869262391 Bhaveshwarikar
Reg. No Name in Full Residential Address Gender Contact No. Email id Remarks 10001 SALPHALE VITTHAL AT POST UMARI (MOTHI) TAL.DIST- Male DEFAULTER SHANKARRAO AKOLA NAME REMOVED 444302 AKOLA MAHARASHTRA 10002 JAGGI RAMANJIT KAUR J.S.JAGGI, GOVIND NAGAR, Male DEFAULTER JASWANT SINGH RAJAPETH, NAME REMOVED AMRAVATI MAHARASHTRA 10003 BAVISKAR DILIP VITHALRAO PLOT NO.2-B, SHIVNAGAR, Male DEFAULTER NR.SHARDA CHOWK, BVS STOP, NAME REMOVED SANGAM TALKIES, NAGPUR MAHARASHTRA 10004 SOMANI VINODKUMAR MAIN ROAD, MANWATH Male 9421864344 RENEWAL UP TO 2018 GOPIKISHAN 431505 PARBHANI Maharashtra 10005 KARMALKAR BHAVESHVARI 11, BHARAT SADAN, 2 ND FLOOR, Female 022 25401313 / bhaveshwarikarmalka@gma NOT RENEW RAVINDRA S.V.ROAD, NAUPADA, THANE 9869262391 il.com (WEST) 400602 THANE Maharashtra 10006 NIRMALKAR DEVENDRA AT- MAREGAON, PO / TA- Male 9423652964 RENEWAL UP TO 2018 VIRUPAKSH MAREGAON, 445303 YAVATMAL Maharashtra 10007 PATIL PREMCHANDRA PATIPURA, WARD NO.18, Male DEFAULTER BHALCHANDRA NAME REMOVED 445001 YAVATMAL MAHARASHTRA 10008 KHAN ALIMKHAN SUJATKHAN AT-PO- LADKHED TA- DARWHA Male 9763175228 NOT RENEW 445208 YAVATMAL Maharashtra 10009 DHANGAWHAL PLINTH HOUSE, 4/A, DHARTI Male 9422288171 RENEWAL UP TO 05/06/2018 SUBHASHKUMAR KHANDU COLONY, NR.G.T.P.STOP, DEOPUR AGRA RD. 424005 DHULE Maharashtra 10010 PATIL SURENDRANATH A/P - PALE KHO. TAL - KALWAN Male 02592 248013 / NOT RENEW DHARMARAJ 9423481207 NASIK Maharashtra 10011 DHANGE PARVEZ ABBAS GREEN ACE RESIDENCY, FLT NO Male 9890207717 RENEWAL UP TO 05/06/2018 402, PLOT NO 73/3, 74/3 SEC- 27, SEAWOODS, -

Naigaon (Khairgaon) District: Nanded
Mudkhed Village Map Takli(T.B.) Dharmabad Taluka: Naigaon (Khairgaon) District: Nanded Vanzirgaon Loha Barbada Umri Mamnyal Manur Tarf Ba Izatgaon (M) Patoda (T.B.) Antargaon Kahala Bk. Izatgaon Bk Sadakpur µ 2.5 1.25 0 2.5 5 7.5 Kahala Kh Mandni km Rui Bk Kushanoor Sawarkhed Rui Kh Sategaon Somthana Location Index Vanjarwadi Ikalimal Dharmabad Babulgaon Ghungrala Melgaon Hiparga (Janerao) Nilegavhan Sangvi Dhanaj District Index Kuntoor Nandurbar Bhandara Narangal Dhule Amravati Nagpur Gondiya Jalgaon Ransugaon Paradwadi Takbid Akola Wardha Hussa Buldana Ancholi Nashik Washim Chandrapur Yavatmal Aurangabad Degaon Charwadi Raher Palghar Salegaon Jalna Hingoli Gadchiroli Kolambi Talbid Takalgaon Thane Ahmednagar Parbhani Mumbai Suburban Nanded Palasgaon Mumbai Bid Godamgaon Kokalegaon Hangraga Raigarh Pune Latur Bidar Lalwandi Osmanabad Awrala Satara Solapur Kauthala Daregaon Naigaonwadi Ratnagiri Shelgaon Chatri Sangli Sujlegaon Maharashtra State Naigaon Kolhapur Manjram Iklimore NAIGAON Sindhudurg Kandhar Bendri !( Dharwad Khairgaon Betak Biloli Manjramwadi Pimpalgaon (Na) Taluka Index Mustapur Mahoor Kinwat Mokasdara Khandgaon Gadga Hotala Kedar Wadgaon Hadgaon Himayatnagar Kopra Narsi Ardhapur Nawandi Bhokar NandedMudkhed Marwali Tanda Loha Umri Aluwadgaon Kandala Biloli Marwali Dharmabad Naigaon (Khairgaon) Kandhar Tembhurni Biloli Legend Mukhed Deglur !( Taluka Head Quarter Dhanora T.M. Ratoli Kuncholi Mugaon Karla T.M. Dhuppa Railway District: Nanded Takli(T.M.) Mahegaon National Highway State Highway Village maps from Land Record Department, GoM. Bhopala Data Source: Shelgaon (Gauri) State Boundary Waterbody/River from Satellite Imagery. District Boundary Generated By: Mukhed Takli Bk. Taluka Boundary Maharashtra Remote Sensing Applications Centre Village Boundary Autonomous Body of Planning Department, Government of Maharashtra, VNIT Campus, Waterbody/River South Am bazari Road, Nagpur 440 010. -

Accumulation of Heavy Metal Ions in Water, Sediment and Aquatic Weeds: a Case Study of Sudha Dam, Bhokar, India
Available online a t www.pelagiaresearchlibrary.com Pelagia Research Library Advances in Applied Science Research, 2013, 4(5):394-400 ISSN: 0976-8610 CODEN (USA): AASRFC Accumulation of heavy metal ions in water, sediment and aquatic weeds: A case study of Sudha Dam, Bhokar, India Mane P. C.1, Kadam D. D.2 and Chaudhari R. D. *2 1School of Earth Sciences, Swami Ramanand Teerth Marathwada University, Nanded(MS), India 2Zoology Research Centre, Shri Shiv Chhatrapati College of Arts, Commerce and Science Junnar, University of Pune, (MS), India _____________________________________________________________________________________________ ABSTRACT The metal pollution in water, sediment and its accumulation in some aquatic weeds of the Sudha dam in Maharashtra (India) was assessed for Cr, Cu, Fe, Mn, Se and Zn. The samples of water, sediment and aquatic weeds were collected at random from different areas of the dam covering all directions. All the samples were analyzed for metal ion concentration using UV-spectrophotometer. The water and sediment samples from the Sudha dam contains considerable amount of the metal ions studied. It has been observed that, in root and leaf all the ions get absorbed, while the extent for each element found different in the parts analyzed. Thus it can be clear that, Typha angustifolia and Vallisneria amricana absorbs heavy metal ions and can be used for minimizing the pollution taking place due to toxic metals from industrial effluents. Key words: Metal pollution, sediment, Typha angustifolia , Vallisneria amricana , UV-spectrophotometer. _____________________________________________________________________________________________ INTRODUCTION In recent years the accumulation of metals in the aquatic ecosystem has become a problem of great concern throughout the world. -

2021011945.Pdf
dmbackof f ice@ gtr9ll!9l1 - w,,vw.nanded.qov ln l F4E -02462245946 qrer, tq{frIfr, i?3 fin-iT:- 18.01.2020 qG({rqT qrfr 'qql S-L stdfrd{lla 5qr 3r{dql cr-s ffii1 ftff q{frdr {ff{ L{il qrqlqrrd qrsnsGdr cias 3d q{frnla Jrfrd di' ar"rff Fdsq6 qdalrfEq i-{6 3 1 . 1 2 2020 irfrrr*d ildT ;r-s roffi erd amtroar alruffivr q?s FT€qrdl-d onqfr s'6tl C€qrd 6ril -le"+5* sG ii-s zozt qfan omEfi 6$dr Tdn=42 elqrd ffi orrqisE EcT- zo ot zozt i Eqia zz ol -air+o qM H5i olqffiq tqr ?tqrslff4 3ld i]{ff ssqd 3rgm E m<slifto ra tqo 'rFoii+ erqd s{6R F{]a E tild iwrf, qfQdr qar' 6Tqf(q {es AE sd' il{{ {rd{ Aad i(€frrfr F lnrf, 6&rr iicfilor{] +r crtrft ii?B qTq 3r"rfd] r5{r d 3'd €rqa sirrqqrar dflflT qTfl in ra €'dl ite nTefalqrErdl qi.s fu6qr4r sqir< €&de'6ral o-sc ?uqm id 3lrA' etge 3rd,anrff ftr€{ar €&€rdr{{ srmqq qFn qTqd 0s% \'{nr ftffi 3{sd"@r s{o]l }3lqr aa.*a q. *ra ftgr]Tsrd 4as c-€qrfid 'd"@l qEdl q4d 10% ?n{d 3lTfrdr qrli€Tqr{fl{ rz1s qq! lT'rrati rTaffd ti]ll1f, .d 3r€d iqrs lqq cdf qfrn filtq ftrcrftroffi aq1 enq& F{6R }-r" zT dr{{a {d .r-r,n c,ii q1,.rqTi {ffd {rdo \d{ vwa i-qeFrda wd e tu6l+dqft-ff, rFr6r+-,rffi+{Itro, a"tII fin6rft#^il?s. -

Umri District: Nanded Bhokar
Village Map Taluka: Umri District: Nanded Bhokar Karla Mudkhed Shivangaon Dholumri Kalgaon µ 2 1 0 2 4 6 Sawargaon (Dakshin) Shirur Palasgaon km Turati Aswaldari Sindhi Mokhandi (Jagir) Durganagar Fulsingnagar Jirona Ishwarnagar Location Index Somthana P.U. Shelgaon Bothi Bitnal Sawargaon (kala) Vasantnagar District Index Ganipur Nandurbar Jamgaon Bhandara Dhule Amravati Nagpur Gondiya Hunda (patti ganga) Gortha Jalgaon UMRI Hunda patti umari Akola Wardha !( Buldana Mandala Nashik Washim Chandrapur Ramkhadak Yavatmal Hiradgaon Kudla Peth Umri (M Cl) Palghar Aurangabad Hunda Tanda Hangiranga Waghala Jalna Hingoli Gadchiroli Thane Ahmednagar Parbhani Telangana State Mumbai Suburban Nanded Mumbai Bid Bolsa Raigarh Pune Abdulapur Latur Bidar Osmanabad Ilegaon P. G. Dilawarpur Satara Solapur Chinchala patti umri Talegaon Dhanora Bk. Ratnagiri Sangli Bhayegaon Manur Maharashtra State Kolhapur Amdapur Sindhudurg Rahati Kh. Dharwad Ijjatgaon Nagthana Kh. Nagthana Bk. Bolsa Kh Bolsa Bk Miyadadpur Taluka Index Mahoor Kinwat Beldara Nimtek Balegaon Hadgaon Himayatnagar Karkala Ardhapur Hatni Bhokar NandedMudkhed Borjuni Loha Umri Mahati Golegaon Waghalwada Dharmabad Naigaon (Khairgaon) Kandhar Biloli Endala Bijegaon Legend Dharmabad Kaudgaon Mukhed Deglur !( Taluka Head Quarter Naigaon (Khairgaon) Railway Hassa District: Nanded Singnapur National Highway Kawalguda Bk. State Highway Village maps from Land Record Department, GoM. Kawalguda kh. Data Source: State Boundary Waterbody/River from Satellite Imagery. District Boundary Generated By: Taluka Boundary Maharashtra Remote Sensing Applications Centre Village Boundary Autonomous Body of Planning Department, Government of Maharashtra, VNIT Campus, Biloli Waterbody/River South Am bazari Road, Nagpur 440 010. -
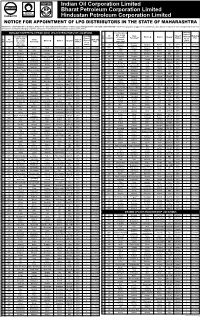
Indian Oil Corporation Limited Bharat Petroleum Corporation Limited Hindustan Petroleum Corporation Limited
Indian Oil Corporation Limited Bharat Petroleum Corporation Limited Hindustan Petroleum Corporation Limited NOTICE FOR APPOINTMENT OF LPG DISTRIBUTORS IN THE State OF MAHARASHTRA INDIAN OIL CORPORATION LTD (IOCL), BHARAT PETROLEUM CORPORATION LTD (BPCL) and HINDUSTAN PETROLEUM CORPORATION LTD (HPCL) proposes to appoint LPG distributors under various categories at locations specified below from amongst the candidates belonging to different categories mentioned therein in the State of (name of the State): DURGAM KSHETRIYA VITRAK (DKV) LPG DISTRIBUTORSHIP LOCATIONS Location (along Security with details Deposit Security Sr. Oil Gram Type of Marketing Location (along of Locality Block* @ District Category** (Amount Deposit No. Company Panchayat* Market Plan with details wherever Rs. in Sr. Oil Gram Type of (Amount Marketing of Locality Block* @ District Category** applicable) Lakhs) No. Company Panchayat* Market Rs. in Plan wherever Chinchave Chinchave Lakhs) 96 IOC Malegaon Nashik SC 2 2017-18 applicable) (Galane) (Galane) DKV 1 IOC Khirvire Khirvire Akole Ahmednagar Open DKV 4 2017-18 97 HPC Nimgaon Nimgaon Malegaon Nashik Open(W) DKV 4 2017-18 2 IOC Hatru Hatru Chikhaldara Amravati OBC DKV 3 2017-18 98 IOC Tingri Tingri Malegaon Nashik Open DKV 4 2017-18 3 IOC Ghodgaon Ghodgaon Chopda Jalgaon ST DKV 2 2017-18 99 BPC Vehelgaon Vehelgaon Nandgaon Nashik OBC DKV 3 2017-18 4 BPC Ghuikhed Ghuikhed Chandur Railway Amravati Open DKV 4 2017-18 100 IOC Thangaon Thangaon Sinnar Nashik ST(W) DKV 2 2017-18 5 IOC Jalalkheda Jalalkheda Narkhed Nagpur -
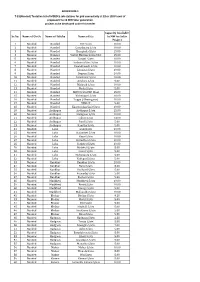
T-31-Nanded.Pdf
ADDENDUM-1 T-31(Nanded) Tentative list of MSEDCL sub-stations for grid connectivity at 22 or 11KV Level of proposed 2 to 10 MW Solar generation projects to be developed under this tender Capacity Available Sr.No. Name of Circle Name of Taluka Name of S/s in MW for Solar Project 1 Nanded Nanded NTC S/stn 10.00 2 Nanded Nanded Gurudwara S/stn 10.00 3 Nanded Nanded Chauphala S/stn 15.00 4 Nanded Nanded Vidyut Bhavan S/stn/CRC 15.00 5 Nanded Nanded Sangvi S/stn 10.00 6 Nanded Nanded Industrial Are S/stn 10.00 7 Nanded Nanded Pawadewadi S/stn 10.00 8 Nanded Nanded Limgaon S/stn 10.00 9 Nanded Nanded Degaon S/stn 10.00 10 Nanded Nanded Naleshwar S/stn 10.00 11 Nanded Nanded Amdura S/stn 5.00 12 Nanded Nanded Matasab S/stn 10.00 13 Nanded Nanded Neela S/stn 5.00 14 Nanded Nanded MIDC S/stn EXP Moal 15.00 15 Nanded Nanded Vishnupuri S/stn 10.00 16 Nanded Nanded Tuppa ( Dhanegaon) 10.00 17 Nanded Nanded MIDC II 5.00 18 Nanded Nanded Kautha (Asarjan) S/stn 10.00 19 Nanded Ardhapur Ardhapur S/stn 15.00 20 Nanded Ardhapur Malegaon S/stn 10.00 21 Nanded Ardhapur Lahan S/stn 14.00 22 Nanded Ardhapur Pardi S/stn 5.00 23 Nanded Ardhapur Kamtha S/stn 5.00 24 Nanded Loha LohaS/stn 10.00 25 Nanded Loha Kalamber S/stn 10.00 26 Nanded Loha Kapsi S/stn 10.00 27 Nanded Loha Shiradhon S/stn 10.00 28 Nanded Loha Sonkhed S/stn 10.00 29 Nanded Loha Malakoli S/stn 8.00 30 Nanded Loha Penur S/stn 5.00 31 Nanded Loha Mohanpura S/stn 5.00 32 Nanded Loha Vadepuri S/stn 5.00 33 Nanded Kandhar Kandhar S/stn 10.00 34 Nanded Kandhar Barul S/stn 8.00 35 Nanded Kandhar Phulwad -

Aqar 2017-2018
Annual Quality Assurance Report (AQAR) Academic Year 2017-18 Submitted by Internal Quality Assurance Cell (IQAC) Digambarrao Bindu Arts, Commerce & Science College Bhokar, Dist. Nanded 431801 Track ID MHCOGN10817 Submitted to NATIONAL ASSESSMENT AND ACCREDITATION COUNCIL An Autonomous Institution of the University Grants Commission P. O. Box. No. 1075, Opp: NLSIU, Nagarbhavi, Bengaluru - 560 072 India To, The Director. National Assessment & Accreditation Council, Post Box. No. 1075, NLSIU, Nagarbhavi, Bangluru, Karnatka 560072, India Subject : Submission of AQAR for the Year 2017-18 Res / Sir With Reference to above cited subject, I am herewith submitting the soft copy of AQAR (Annual quality Assurance Report) for the year 2016- 17. Kindly consider same and oblige. Enclosure : 1. Copy of the AQAR (Annual quality Assurance Report) Date : 30.06.2018 Seal Principal The Annual Quality Assurance Report (AQAR) of the IQAC Part – A AQAR for the year : 2017-18 1. Details of the Institution 1.1 Name of the Institution : Digambarrao Bindu Art‟s, Commerce & Science College 1.2 Address : Tamsa Road, Bhokar, Dist. Nanded State Maharashtra Pin Code : 431801 Institution e-mail address : [email protected] Contact Nos. : 02467-222892 Name of the Head of the Institution : Principal Dr. Panjab A. Chavhan Tel. No. with STD Code : 02467-222892 Mobile : 9405384251 Name of the IQAC Co-ordinator : Dr. Arvind B. Chavhan Mobile : 9420775527 IQAC e-mail address : [email protected] 1.3 NAAC Track ID : MHCOGN10817 1.4 NAAC Executive Committee : EC(SC)/15/A&A/39.2 dated 25/05/2016 No. & Date. 1.5 Website address : www.dbcbhokar.in Web-link of the AQAR : http://www.dbcbhokar.in/aqar-2017-18.html 1.6 Accreditation Details Year of Sl. -
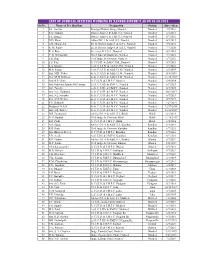
List of Judicial Officers Working in Nanded District As on 25-11-2019
List of JudiciaL officers Working in nanded district as on 01-08-2021 Sr.No. Name of J.O. Shri/Smt. Designation Station Since when 1 S.L. Anekar Principal District Judge, Nanded Nanded 7/2/2021 2 K.N. Gautam District Judge-1 & Addl. S.J., Nanded Nanded 6/4/2018 3 S.E. Bangar District Judge-2 & Addl. S.J., Nanded Nanded 6/7/2021 4 N.D. Khose Adhoc D.J.-1 & Addl. S.J., Nanded Nanded 6/3/2019 5 A.R. Dhamecha Ex. Jt. District Judge-1 & A.S.J., Nanded Nanded 9/5/2018 6 R. M. Pande Ex. Jt. District Judge-1 & A.S.J., Nanded Nanded 7/7/2020 7 R. S. Rote Secretary, D.L.S.A., Nanded Nanded 6/3/2019 8 Y. K. Rahangdale Chief Judicial Magistrate, Nanded Nanded 7/1/2021 9 S.B. Dige Civil Judge Sr. Division, Nanded Nanded 6/7/2021 10 S.S. Patil Jt. C.J.S.D. & Addl.C.J.M., Nanded Nanded 6/3/2019 11 S.A. Khalane 2nd Jt. C.J.S.D. & Addl.C.J.M., Nanded Nanded 6/7/2021 12 M.P. Shinde 3rd Jt. C.J.S.D. & Addl.C.J.M., Nanded Nanded 6/3/2019 13 Smt. M.R. Yadav 4th Jt. C.J.S.D. & Addl.C.J.M., Nanded Nanded 11/8/2019 14 Smt. M. B. Kulkarni 5th Jt. C.J.S.D. & Addl.C.J.M., Nanded Nanded 11/18/2019 15 Smt. R.P. Ghole Jt. C.J.J.D. -

COVID-19 Second Wave: Challenges for Sustainable Development (CCSD 2021) Jointly Organized By
AATETE NEWNEW DD September 13th, 14th & 15th, 2021 Award Ceremony & International Web Conference COVID-19 Second Wave: Challenges for Sustainable Development (CCSD 2021) Jointly organized by Asian Biological Research Foundation (ABRF) Samrat Prithviraj Chauhan Government College Prayagraj (U.P.), India Ajmer (Rajasthan) http://www.abrf.org.in https://hte.rajasthan.gov.in/college/gcajmer Govt. KRG Post Graduate (Autonomous) College Govt. Degree College Gwalior (M.P.) Doda (J.& K.) http://krgcgwalior.org/ https://www.gdcdoda.com/ Digambarrao Bindu Arts, Commerce & Science College Darsh College of Education Bhokar Dist. Nanded (Maharashtra) Gohana, Sonepat (Haryana) https://www.dbcbhokar.in/ http://www.darsh.edu.in/ Glocal Environment & Social Association (GESA) New Delhi http://www.gesa.org.in IMPORTANT l Platform: Zoom app and YouTube. l PDF certificate will be sent through email to all registered participants. l Conference Registration fee: Rs. 200 for Faculty/Academician/Scientist and Rs. 100 for Research Scholar. l Free for PG students; Registration not required. l E-abstract book will be provided. l Registered at: https://forms.gle/8eyriEt1Nn3ANpdk7 l Timing: 4 pm to 7 pm on 13th, 14th & 15th, Sep. 2021 l Email id: [email protected] l Zoom app link: https://us02web.zoom.us/j/88644507903?pwd=a3VmOEtiak8yenJMbSsxSE1 wMDl4UT09 l YouTube link: Day 1: https://youtu.be/_wvA2P-HLUw Day 2: https://youtu.be/gRyAzKqAfso Day 3: https://youtu.be/fuzsoNVcGqg l Contact person for awards: Mr Prabhakar Singh Ph. 9452662412 l Phone: 8299707543 (for general enquiry) Call for Book Chapters/manuscripts Dear Researcher, We are pleased to invite you and other esteemed colleagues to contribute a book chapter in the form of research papers, review articles, special notes, survey reports, etc. -

Village Map Taluka: Deglur District: Nanded
Biloli Village Map Manshakarga Taluka: Deglur Apsavargaon Shevala Lakhkha District: Nanded Sugaon Kotekallur Wazarga Shekhapur Nipani Savargaon Limba Nandur Tupshelgaon Alur Ibrahimpur Wannali Rampur P.shahapur Shelgaon Shahapur Alapur Khanapur Antapur Kare Malkapur Chainpur Sujayatpur Tamlur Tadkhel µ 3 1.5 0 3 6 9 Malegoan Munjalga Narangal Bk. Mendankallur km Mukhed Takli Bagam Bomnali Thadi Sawargaon HawargaSundgi Bk. Sangvi Umar Degaon Bk. Degaon Kh Hanuman Hipparga Mandgi Gap_das Sundgi Kh. Kavalgadda DEGLUR Narangal Kh Bhayegaon !( Kurudgi Bk Shiv Achegaon Malkapur Takli Walag Location Index Achegaon Deglur (M Cl) Dhosni Karegaon Bhokaskheda Balegaon Chakur Mailapur (D) Pimpalgaon District Index Nandurbar Kavalgaon Bhandara Amravati Lingankerur Dhule Nagpur Gondiya Mengapur (D) Ballur Jalgaon Akola Wardha Daregaon Sangvi Karadkhed Rampur Bk. Buldana Borgaon Nashik Washim Chandrapur Wadikarkhed Bhaktapur Yavatmal Palghar Aurangabad Amdapur Kutub Shahapurwadi Jalna Gadchiroli Nagral Hingoli Gavandgaon Thane Ahmednagar Parbhani Karadkhed Mumbai Suburban Nanded Hotal Mumbai Bid Kathewadi Raigarh Pune Naiktanda (N.V.) Latur Bidar Osmanabad Walag Kedarkunta Solapur Shivani Satara Devapur Ratnagiri Sangli Pendpalli Yergi Maharashtra State Markhel Zari Kolhapur Udgir Sindhudurg Dharwad Takali Jahagir Davangir Malegaon (M) Taluka Index Mahoor Kinwat Pujarwadi Kini Kinitanda Kshirsamudra Mangajiwadi Ambulga Hadgaon Loni Himayatnagar Tumbarpalli Martoli Ardhapur Hali Bhokar NandedMudkhed Bhutan Hipparga Loha Umri Dharmabad Shilwani Chavanwadi