CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
State Zone Commissionerate Name Division Name Range Name
Commissionerate State Zone Division Name Range Name Range Jurisdiction Name Gujarat Ahmedabad Ahmedabad South Rakhial Range I On the northern side the jurisdiction extends upto and inclusive of Ajaji-ni-Canal, Khodani Muvadi, Ringlu-ni-Muvadi and Badodara Village of Daskroi Taluka. It extends Undrel, Bhavda, Bakrol-Bujrang, Susserny, Ketrod, Vastral, Vadod of Daskroi Taluka and including the area to the south of Ahmedabad-Zalod Highway. On southern side it extends upto Gomtipur Jhulta Minars, Rasta Amraiwadi road from its intersection with Narol-Naroda Highway towards east. On the western side it extend upto Gomtipur road, Sukhramnagar road except Gomtipur area including textile mills viz. Ahmedabad New Cotton Mills, Mihir Textiles, Ashima Denims & Bharat Suryodaya(closed). Gujarat Ahmedabad Ahmedabad South Rakhial Range II On the northern side of this range extends upto the road from Udyognagar Post Office to Viratnagar (excluding Viratnagar) Narol-Naroda Highway (Soni ni Chawl) upto Mehta Petrol Pump at Rakhial Odhav Road. From Malaksaban Stadium and railway crossing Lal Bahadur Shashtri Marg upto Mehta Petrol Pump on Rakhial-Odhav. On the eastern side it extends from Mehta Petrol Pump to opposite of Sukhramnagar at Khandubhai Desai Marg. On Southern side it excludes upto Narol-Naroda Highway from its crossing by Odhav Road to Rajdeep Society. On the southern side it extends upto kulcha road from Rajdeep Society to Nagarvel Hanuman upto Gomtipur Road(excluding Gomtipur Village) from opposite side of Khandubhai Marg. Jurisdiction of this range including seven Mills viz. Anil Synthetics, New Rajpur Mills, Monogram Mills, Vivekananda Mill, Soma Textile Mills, Ajit Mills and Marsdan Spinning Mills. -
Branch List ACPC 2018.Xlsx
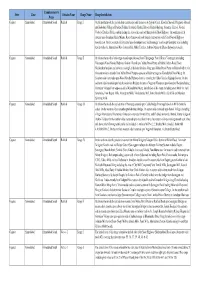
KOTAK MAHINDRA BANK BRANCH FOR PIN DISTRIBUTION DISTRICT CITY BRANCH NAME ADRESS OF THE BANK BRANCH Kotak Mahindra Bank Ltd. Ground Floor,Chandan House,Opp. Abhijeet Iii,Near Ahmedabad Ahmedabad Navrangpura Mithakali Six Roads,Navrangpura, Ahmedabad,Gujarat - 380 009 Tel No. 079).66614800 Kotak Mahindra Bank Ltd. Shop No. 6 & 7, Sidhivinayak Complex, Shivranjini Char Rasta, Ahmedabad Ahmedabad Satelite Satellite, Ahmedabad, Gujarat - 380015 Tel - (079) 66319151 Kotak Mahindra Bank Limited Prime Plaza, Ground Floor, Opp. Rajiv Bhai Tower, Ahmedabad Ahmedabad Maninagar Maninagar, Ahmedabad - 380008 Tel No. (079) 66060265 / 66 Kotak Mahindra Bank Ltd. 1st Floor, Shyamal Ahmedabad Ahmedabad Chandkheda Comlpex, New C.G.Road,Chandkheda, Ahmedabad,Gujarat - 382 424. Kotak Mahindra Bank Ltd. Block B ,39 to 42"Tejendra" Complex,opp Torrent power Ahmedabad Ahmedabad Odhav station, Soni ni chal, Odhav Road, Ahmedabad. Pin Code - 382415 Kotak Mahindra Bank; “Satvad Complex”, Ahmedabad Ahmedabad Naranpura Sardar Patel Stadium Road,Naranpura Ahmedabad.Gujarat. Pin Code - 380 013 Kotak Mahindra Bank."Prime Plaza” Satya Ahmedabad Ahmedabad Bodakdev Marg, Judges bungalow Road, Bodakdev, Ahmedabad. Pin Code - 380054 Kotak Mahindra Bank Ltd. Ground Floor And 1St Floor,Parekh Chambers, Near Small Bus Amreli Amreli Amreli Stop, Dr. Jivraj Mehta Chowk, Amreli - 365 601. Tel - 9228006017 Kotak Mahindra Bank Ltd. P M Chambers, Opp Anand Vallabhvidyanagar Vallabhvidyanagar Lucky Auto Center, Mota Bazar, Vallabh Vidya Nagar, Gujarat - 388 120 Kotak Mahindra Bank Ltd. Ground Floor, Banas Kantha Palanpur Palanpur Agrawal Complex, Palace Road, Palanpur - 385 001 Tel - (02742) 652627 To 35 KOTAK MAHINDRA BANK BRANCH FOR PIN DISTRIBUTION DISTRICT CITY BRANCH NAME ADRESS OF THE BANK BRANCH Kotak Mahindra Bank Ltd. -

Updated Admin Contact List of All Covid-19 Pvt Hospitals As on Date 03/05/2021
UPDATED ADMIN CONTACT LIST OF ALL COVID-19 PVT HOSPITALS AS ON DATE 03/05/2021 SR. NO. ZONE HOSPITAL NAME NODAL PERSON MOBILE NO. 1 WZ / CHANDKHEDA SMS HOSPITAL DR. CHAITRY 99241 10197 2 WZ / CHANDKHEDA NAVJIVAN DARSHANBHAI 80000 81848 3 WZ/ NAVRANGPURA HCG HOSPITAL, MITHAKALI DR VASANT PATEL 75730 42831 4 WZ / NAVARANGPURA AARTHAM HOSPITAL, AMBAWADI, DR. SANSKAR DAVE 90239 71118 PURVANG BHAI 73590 33305 WZ / NAVRANGPURA SUSHRUSHA", NAVRANGPURA 5 DR ANIS SHAH 98250 22685 6 WZ / NAVRANGPURA NIDHI HOSPITAL, NAVRANGPURA DR.TAPAN DAVE 98799 61261 7 WZ / NAVRANGPURA TURNING POINT HRIDAY SE KINJALBEN 98985 15785 DR SHAILESH SHAH 98240 35673 WZ / PALDI BODYLINE HOSPITAL 8 ALOK BHADORIYA 98240 76704 9 WZ / USMANPURA MANSAROVAR DR. YOGESH PUROHIT 98796 97744 TRISHA MULTI SPECIALITY, WZ / NAVA WADAJ DR HITESH 94265 17989 10 NIRNAY NAGAR 11 WZ / CHANDKHEDA LOTUS DR.MIKY PATEL 99090 07305 12 WZ / CHANDKHEDA TLGH DR.SANDIP 98981 22262 13 WZ / NARANPURA GURU PREM DR.SATISH CHIKALKAR 70710 80108 14 WZ / NAVA VADAJ CARE PLUS DR. VIKRAM PATEL 98253 93738 15 WZ / CHANDKHEDA RAJDEEP HOSPITAL DR. ROHAN 98797 35982 16 WZ / NARANPURA SHAILYA CHRISTIAN VICKY 95123 66060 17 WZ/ CHANKHEDA ANSH HOSPITAL DR NITIN PRAJAPATI 99790 55875 18 WZ / NAVA VADAJ ATHARVA HOSPITAL DR. PRATIK THAKKAR 99784 88967 19 WZ / CHANDKHEDA SATYAMEV DR. SANDESARA 98790 74447 20 WZ / CHANDKHEDA SENTARA HOSPITAL DR.JENISH PATEL 80002 12142 21 WZ / NAVRANGPURA SHALBY, NAVRANGPURA DR. DIPAK 78528 98215 22 WZ / NARANPURA SOLAR HOSPITAL, NARANPURA, DR KAUSHIK GOSWAMI 99091 95940 MAULIK PATEL 99042 66999 WZ/ NAVA VADAJ MANSI HOSPITAL 23 DR. MANSI PATEL 97125 59375 24 WZ / VASNA PARTH HOSPITAL DR. -

Company List
28 views 0 0 RELATED TITLES Company List Uploaded by Dharmesh Gohel name of companies china Full description Save Embed Share Print gheekanta.pptx Steve Jobs 1902184128AFD exempted_est_list.pdf DEALER LIST- 19 Company_List www.fundoodata.com Accusol Technologies Pvt. Ltd.(Pending) Address: B-201, Safal Pegasus, Anand Nagar Road. Nr. Prahlad Nagar Garden, Ahmedabad-380051 Phone: +91 79 4003 7105 Mobile: +91 96876 12255, +91 99256 33388, +91 99789 60634 Email: Sales: [email protected] Jobs: [email protected] Info: [email protected] Website : http://www.teratechnolabs.com/ -- 28 views 0 0 RELATED TITLES Company List Uploaded by Dharmesh Gohel name of companies china Full description Save Embed Share Print gheekanta.pptx Steve Jobs 1902184128AFD exempted_est_list.pdf DEALER LIST- 19 205, 2nd Floor, Ashwamegh Avenue, Nr - Mithakali Underbridge Navrangpura, Ahmedabad - 380009 Recruiter Name : Sanjay Delvadiya Email Address : [email protected], [email protected] Send your resume on mentioned Email address with your current company details and current 306, B-Wing Gopal palace, Opp. Ocean Park, Nehrunagar, Satelite, Ahmedabad - 380001. Gujrat, India. India: +91 (740) 5256 168 General: [email protected] 28 views 0 0 RELATED TITLES Company List Uploaded by Dharmesh Gohel name of companies china Full description Save Embed Share Print gheekanta.pptx Steve Jobs 1902184128AFD exempted_est_list.pdf DEALER LIST- 19 Careers: [email protected] www.bitplus(pending) www.abbacus technologies.com contact no:079 65121731 www.shine.com(register for job) id:[email protected] BrainFeed Solutions 404 Earth Complex, Nr. Seema Hall, Anandnagar, Ahmedabad 380 015, Gujarat, India. Phone*: +91 (079) 400 60 776 Mobile*: +91 9898 6666 00 Email: [email protected] Concept Infoway Private Limited 801 B Parshwa Tower, S.G. -

1945 , 16/03/2020 Class 35 2592012 05/09/2013 Trading As
Trade Marks Journal No: 1945 , 16/03/2020 Class 35 2592012 05/09/2013 SURESH KUMAR AGARWAL trading as ;DAZZLES 112, BANGUR AVENUE,BLOCK-D,SHOP NO.03,KOLKATA 700055 SERVICE PROVIDER. Address for service in India/Agents address: SHUKLA TRADE MARK COMPANY. 71, CANNING STREET, ROOM NO. C - 518, 5TH FLOOR, BAGRI MARKET, KOLKATA- 700 001. Used Since :07/04/2010 KOLKATA Advertising, Distribution, Marketing, Wholesale, Departmental Store and trading services relating to Retail Showroom and Exclusive Showroom and other establishment for sale of Ladies Garments; Salwar Suits & Kurties included in class-35. 3534 Trade Marks Journal No: 1945 , 16/03/2020 Class 35 2720475 17/04/2014 VEDANT FASHIONS PRIVATE LIMITED at PARIDHAN GARMENT PARK,19, CANAL SOUTH ROAD SDF-1, 4TH FLOOR,A 501-502,KOLKATA-700015 WEST BENGAL SERVICE Address for service in India/Agents address: S. MAJUMDAR & CO. 5, HARISH MUKHERJEE ROAD, KOLKATA - 700 025, INDIA. Used Since :03/12/2001 To be associated with: 1774050, 2701680, 2701681 KOLKATA Services rendered in India and for export to other countries for retailing and wholesaling, including online retailing and wholesaling services; e commerce, retail store outlets; bringing together, for the benefit of others, of a variety of goods enabling customers to conveniently view and purchase those goods including services relating readymade garments of all kinds, headgear, boots, shoes and slippers and accessories thereof in class no. 35 3535 Trade Marks Journal No: 1945 , 16/03/2020 Class 35 2735437 12/05/2014 HINESH M. RUGHANI RAKESH V. KHIRA trading as ;MICRO IMPEX SHOP NO. 3, OLD HANUMAN BUILDING, GR. -

Corrigendum 4 Tender No: 1 Smart City Ahmedabad Development Ltd (SCADL) 241853
Smart City Ahmedabad Development Limited CEO, Smart City Ahmedabad Development Ltd (SCADL), Ahmedabad Municipal Corporation, Sardar Patel Bhavan, Danapith, Ahmedabad, . Gujarat 380001 Corrigendum 4 Tender No: 1 Smart City Ahmedabad Development Ltd (SCADL) 241853 241853- Notice Inviting RFP for Selection of Implementation Agency for Supply, Installation, Commissioning and operation & maintenance of Pan city ICT Infrastructure and Integrated Command and Control Center for Smart City Ahmedabad (Gujarat) – Smart city Ahmedabad Development Ltd(SCADL) Post due considerations to the queries received this corrigendum has been consequently released. # Information Details 1 Online Price Bid Submission Date 27/03/2017 up to 17:00 Hrs. In Sealed envelope strictly by RPAD/ Postal Speed Post/ Courier on or before 29/03/2017 17:00 Hrs. to Technical Bid Submission (in Hard Copy) CEO Smart City Ahmedabad Development Ltd 2 Filled-in Technical Bid along with Bid (SCADL), Ahmedabad Municipal Corporation, Sardar Fee, EMD and other documents Patel Bhavan, Danapith, Ahmedabad, Gujarat 380001 with necessary documents (Tender Fee, EMD, etc.) as mentioned in the RFP 3 Date & Time of opening of Technical To be intimated to the qualified bidders & Commercial bid 4 Contact person and email id [email protected] Consultant: Responses to the queries Page No./ # Clause Reference Query Response to Query Section 1 Section 18: Image sensor : 1/2.7" Request to accept 1/2.8" or better as 1/2.7" is Specification Revised as " Image Technical & Progressive Scan -

The Sandesh Limited
THE SANDESH LIMITED CIN: L22121GJ1943PLC000183 REGD. OFFICE :‐ "SANDESH BHAVAN", LAD SOCIETY ROAD, B/H. VASTRAPUR GAM, P.O. BODAKDEV, AHMEDABAD‐380 054 (GUJ.) UNPAID DIVIDEND FOR THE F.Y. 2016-2017 AS ON 05.05.2017 FOLIO NO. CLIENT ID/ DP ID NAME OF THE SHAREHOLDERS ADRESS OF SHAREHOLDERS PINCODE NETAMT 00004001 AMULAKH PRAHLADJI BRAHMBHATT P.BOX NO.1857. DARESALAM. TANZANIA (AFRICA) TANZANIA (AFRICA) 999999 1500.00 00004002 VINUBHAI M. PATEL 76 FERNE PARK ROAD HORNSEY LONDON N.8 (U.K) LONDON (U.K) 999999 13500.00 00006874 ANILABEN SHAH 1060 RAGHUNATH BUMB'S POLE SANKDI SHERI RAIPUR AHMEDABAD 380001 250.00 00015001 RATILAL NATHALAL SHARDA MANDIR ELLISBRIDGE AHMEDABAD 380006 1500.00 00015015 JASHVANTLAL MANILAL SETH SHAHIBAUG AHMEDABAD 380004 3750.00 00015018 BHANJI DURLABHJI SHAH NEAR PANJRA POLE RATANPOLE AHMEDABAD 380001 750.00 00015027 CHANDRAMANI RAVJIBHAI AMIN OPP.KUMKUM BUNGLOW JAINMANDIR ROAD MANINAGAR AHMEDABAD 380008 750.00 00015028 BHAGVANDAS TULSIDAS ELECTRIC FOREMAN THE N. R. C. LTD. P. O. KALYAN (G.I.P.) KALYAN 421301 3000.00 00015047 VINODCHANDRA JESHINGBHAI SHAH VIDYANAGAR SOCIETY NR.GUJARAT VIDYAPITH ASHRAM ROAD AHMEDABAD 380013 1500.00 00015048 SHANTAGAURI VYAS 1646.SEWKA'S WADI KHADIA AHMEDABAD 380001 750.00 00015049 PURUSHOTAMDAS ISHWARBHAI PATEL SHRI NARAYANKUNJ LALLUBHAI'S POLE OUTSIDE SHAHPUR DARWAJA AHMEDABAD 380001 750.00 00015050 CHHOTALAL MULJIBHAI B PATEL C/O PURUSHOTAMDAS DHORIBHAI AND CO TARCHHAP BIDI WORKS DHAMTARI-49 3 773 DIST.RAIPUR(M.P) DHAMTARI 493773 750.00 00015053 CHANDULAL KESHAVLAL MODI NEAR BHANDERI POLE INSIDE KALUPUR DARWAJA AHMEDABAD 380001 750.00 00015061 SHRIMANPRASAD GORDHANDAS BHOIWADA'S POLE KALUPUR AHMEDABAD 380001 750.00 00015062 BHADRABEN BALVANTRAY MUNSHI C/O SHRI N.C.DESAI 43.SAMASTA BRAHAMKSHATRIYA SOC NR CHANDRA NAGAR BUS STAND AHMEDABAD 380007 750.00 00015068 GHANSHYAM MANILAL SHAH 8, FALGUN SOCIETY B/H. -

Placement & Hr Consultants
PLACEMENT & HR CONSULTANTS Address Name and Information 1. Benchmark HR Solutions (India) Pvt. Ltd. 2nd Floor, B-Block, Benchmark HR Solutions is leading recruitment consultant company based in Ahmedabad, Swiss Plaza, Gujarat, India. Benchmark HR Solutions offers Opposite Manikbaug Hall, services of recruitment and selection, skill Nehrunagar, enhancement and training, payroll processing, Ahmedabad - 380009 management consultancy. Tel:+(91)-(79) - 40225511, http://benchmarkhrsolutions.com/ 9925069999 2. Career Search Consultants It is a Young, upcoming, professional, Suite #108, Management Consultants having the right Nandan Complex expertise & hands on experience in various Opp. Mithakali Gam fields and industries. Railway Crossing, http://www.indiun.com Behind Natraj Cinema, Mithakali Ahmedabad - 380006 Tel:+(91)-(79) - 9825083474 3. Prime Placement & Manpower Services B- 5, Jayraj Complex, 2Nd It is a placement agency providing recruitment Floor, Soni Ni Chali 4 Rasta, service to corporates in various segments. Odhav Road Ahmedabad - 382415 Tel:+(91)-(79) - 65134568, 66615545 4. Pidison Consultants Established in 1995, Pidison is one of the B-5, (Basement) leading placement services provider in Mahakant Building, Ahmedabad. It provides high quality human Opposite V.S. Hospital, man power services in India and abroad. Ellis bridge, http://www.pidison.com/ Ashram Road, Ahmedabad - 380 006 Tel:+(91)-(79) - 26581371 5. Job's Valley One of the leading placement consultancy 303, Aarohi Complex, service provider, Job's Valley offer placement in Nr. Rasranjan, the areas of Finance, Banking, Telecom, Vijay cross roads, Service, FMGC and many more. Navrangpura, http://www.jobsvalley.com/ Ahmedabad - 380 009 Tel:+(91)-(79) - 27913650 6. High Heads Management Consultants A fast and reliable placement service provider, 218, 4th Floor, High Heads provide placement services which Moonlight Complex, is best suited to the needs of your organization. -
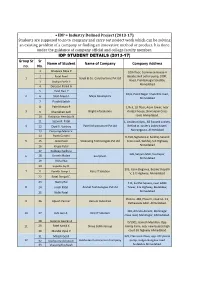
Group Sr No. Sr No. Name of Student Name of Company Company Address • IDP = Industry Defined Project (2013-17) Students Are Su
• IDP = Industry Defined Project (2013-17) Students are supposed to go to company and carry out project work which can be solving an existing problem of a company or finding an innovative method or product. It is done under the guidance of company official and college faculty member. IDP STUDENT DETAILS (2013-17) Group Sr Sr Name of Student Name of Company Company Address no. No. 1 Ahalpara Nilpa P 10th floor, Commerce House-4 2 Patel Fenil Beside shell petrol pump, 100ft. 1 Goyal & Co. Constructions Pvt Ltd 3 Dodiya Parth P Road, Prahladnagar Satellite, Ahmedabad 4 Derasari Pankil A 5 Patel Ravi P 10/A, Punit Nagar-3 Satellite road, 2 6 Shah Meet A Maya Developers Ahmedabad 7 Purohit Satish 8 Patel Himani P 1/A-3, 1st floor, Arjun tower, near 3 9 Khambhati Saifi Bright Infosolution chiripal house, Shivranjani Cross 10 Kodiyatar Hemlata N road, Ahmedabad. 11 Sajani R. Patel 1, Anubhuti Apts., 82 Swastik society, 4 12 Dipti V. Vaishay Patel Infrastructure Pvt Ltd Behind st. xavier's ladies hostel, 13 Pansuriya Mansi V. Navrangpura, Ahmedabad 14 Parth Gandhi D-910, Signature-2, Sarkhej-Sanand 5 15 Janki Patel Silverwing Technologies Pvt Ltd Cross road, Sarkhej, S.G Highway 16 Krupa Patel Ahmedabad 17 Kuldeep Gadhavi 109, Satyam Mall, Vastrapur, 6 18 Urvesh Malani SculptSoft Ahmedabad 19 Vishal Nai 20 Supeda Jay D. 503, Iscon Elegance, Beside Shapath 7 21 Parekh Dimpi T. Kare IT Solution V, S.G Highway, Ahmedabad 22 Patel Dimpal C. 23 Nancy Pal 411, Sarthik Square, near GNFC 8 24 Jinish Patel Aruhat Technologies Pvt Ltd Tower, S.G Highway, Bodakdev, 25 Nidhi Patel Ahmedabad Plot no. -
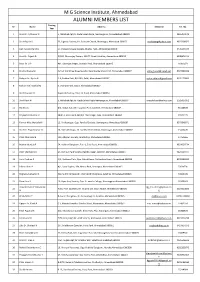
ALUMNI MEMBERS LIST Passing Re Name Address E Mail ID Tel
M G Science Institute, Ahmedabad ALUMNI MEMBERS LIST Passing Re Name Address E Mail ID Tel. No. Year 1 Shah Dr. Ajitkumar P. 5, Abhishek Apt;Nr. Dada Saheb Pagla, Navrangpura, Ahmeddabad-380009 9825019572 2 Shah Rajesh V. 33, Uganda Society, Nr. Subhash Chawk, Memnagar, Ahmeabad-380052 [email protected] 9825008925 3 Dani Sureshchandra. 17, Dungarshinagar Society, Bhatta, Paldi, Ahmedabad-380007 9426543344 4 Shah Dr. Rajesh D. D/502, Dhananjay Towers, 100 FT. Road, Satellite, Ahmedabad-380015 9898874111 5 Patel Dr. V.P. 401, Saraswati Nagar, Himatlal Park, Ahmedabad-380015 26302871 6 Shukla Alpana M. Ashish' 14, Shree Nivas Society, New Sharda Mandir Rd. Ahmedabad-380007 [email protected] 9327000264 7 Rafique Dr. Aysha N. 5-A, Prabha Park, B/H NID, Paldi, Ahmedabad-380007 [email protected] 9376179846 8 Mehta Prof. Rasiklal M 9, Vishram Park, Vasna, Ahmedabad-380007 9 Shah Paulomi H Gopinath Society, Drive-in-Road, Ahmedabad-380054 10 Shah Karvi N 5, Abhishek Apt; Nr. Dada Saheb Pagla,Navrangpura, Ahmedabad-380007 [email protected] 9925010951 11 Rai Hita S. B/2, Adesh Apt; B/h Hasubhai Park, Satellite, Ahmedabad-380007 55120678 12 Prajapati Babubhai B 38/413, Greenpark Apt;B/h. Parasnagar, Sola, Ahmedabad-380062 27412175 13 Parmar Miss Manjula M 12, Vinabanagar, Opp. Panchsil Society, Usmanpura, Ahmedabad-380007 9375948071 14 Shah Dr. Piyushkumar N 28, Shrinath Krupa, Nr. Sarddar Patel School, Maninagar, Ahmedabad-380007 25463470 15 Patel Chimanlal R 123, Alkapuri Society, Ghatlodiya, Ahmedabad-380061 27454665 16 Madan Manjula P 54, Vaibhav Bunglows, Part-1, Sola Road, Ahmedabad-380061 9824050734 17 Patel Shantaben K 24, Jodhpur Park Society, Ramdevnagar, Satellite, Ahmedabad-380015 9825253977 18 Joshi Pratima G A/3, Rainbow Flats, Opp. -

Trade Marks Journal No: 2000 , 17/05/2021 Class 35 2883726 16/01/2015 Address for Service in India/Attorney Address: MU
Trade Marks Journal No: 2000 , 17/05/2021 Class 35 2883726 16/01/2015 M/S.SHIVAM BETELNUT PVT LTD. A- 204A, IIND FLOOR, NORTH EX MALL,, SECTOR-9, ROHINI,DELHI - 110085,DELHI, INDIA SERVICE PROVIDER Body incorporate Address for service in India/Attorney address: CHANDRAKANT & ASSOCIATE CHHATRAPATI SHIVAJI RAJE COMPLEX, BUILDING NO.6, FLAT NO.5, GROUND FLOOR, OPP. EKTA NAGAR, KANDIVALI (WEST), MUMBAI-400 067. Used Since :01/01/2015 MUMBAI ADVERTISING, BUSINESS MANAGEMENT, BUSINESS ADMINISTRATION, OFFICE FUNCTIONS, WHOLESALING, TRADING, DISTRIBUTION, RETAILING AND IMPORT-EXPORT SERVICES IN RESPECT OF KHAINI, FILTER KHAINI, TOBACCO, GUTKHA (TOBACCO), MEETHI SUPARI, MOUTH FRESHENER AND PAN MASALA IN CLASS-35 THE GOODS/SERVICES FOR SALE/CONDUCT IN THE STATES OF Delhi.. 4486 Trade Marks Journal No: 2000 , 17/05/2021 Class 35 3140724 29/12/2015 KAILASH GANESH DESHMUKH SHAHU NAGAR, TANAJI MARG, NEAR HANUMAN TEMPLE, BEED, MAHARASHTRA 431122 SERVICE PROVIDER INDIAN NATIONAL Proposed to be Used MUMBAI ADVERTISING; BUSINESS MANAGEMENT; BUSINESS ADMINISTRATION; OFFICE FUNCTIONS 4487 Trade Marks Journal No: 2000 , 17/05/2021 Class 35 3181388 10/02/2016 JAYESH RAGHU PATEL trading as ;SHREEDHAM SWEETS AND DRYFRUITS SHOP NO.07, ROSARIO C.H.S. LTD, I.C. COLONY, NEAR CITIZEN BANK, BORIVALI (W), MUMBAI-400103. MAHARASHTRA, (INDIA). SERVICE PROVIDERS AN INDIAN NATIONAL Address for service in India/Attorney address: CHANDRAKANT & ASSOCIATE CHHATRAPATI SHIVAJI RAJE COMPLEX, BUILDING NO.6, FLAT NO.5, GROUND FLOOR, OPP. EKTA NAGAR, KANDIVALI (WEST), MUMBAI-400 067. Used Since :01/01/2010 MUMBAI RETAIL SHOP RELETED TO SWEETS & DRYFRUITS AND CONFECTIONARY. THE GOODS/SERVICES FOR SALE/CONDUCT IN THE STATES OF MAHARASHTRA. -
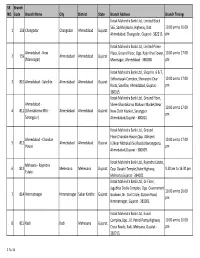
Bank Branch List with Address
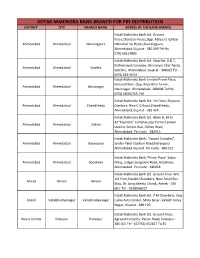
SR Branch NO. Code Branch Name City District State Branch Address Branch Timings Kotak Mahindra Bank Ltd., Limited Block 565, Sarkhej Bavla, Highway, Dist. 10.00 am to 16.00 1 158 Changodar Changodar Ahmedabad Gujarat Ahmedabad, Changodar, Gujarat - 382213. pm Kotak Mahindra Bank Ltd., Limited Prime Ahmedabad - New Plaza, Ground Floor, Opp. Rajiv Bhai Tower, 10.00 am to 17.00 2 159 Ahmedabad Ahmedabad Gujarat (Maninagar) Maninagar, Ahmedabad - 380008. pm Kotak Mahindra Bank Ltd., Shop No. 6 & 7, Sidhivinayak Complex, Shivranjini Char 10.00 am to 17.00 3 810 Ahmedabad - Satellite Ahmedabad Ahmedabad Gujarat Rasta, Satellite, Ahmedabad, Gujarat - pm 380015. Kotak Mahindra Bank Ltd., Ground Floor, Ahmedabad - Shree Ghantakarna Mahavir Market,Near 10.00 am to 17.00 4 811 (Ghantakarna Mkt.- Ahmedabad Ahmedabad Gujarat New Cloth Market, Sarangpur pm Sarangpur) Ahmedabad,Gujarat - 380002. Kotak Mahindra Bank Ltd., Ground Floor,Chandan House,Opp. Abhijeet Ahmedabad - Chandan 10.00 am to 17.00 5 812 Ahmedabad Ahmedabad Gujarat Iii,Near Mithakali Six Roads,Navrangpura, House pm Ahmedabad,Gujarat - 380009. Kotak Mahindra Bank Ltd., Rajendra Estate, Mehsana - Rajendra 6 813 Mehesana Mehesana Gujarat Opp. Gayatri Temple,State Highway, 9.30 am to 16.30 pm Estate. Mehsana,Gujarat - 384002. Kotak Mahindra Bank Ltd., Gr.Floor, Jagubhai Dodia Complex, Opp. Government 10.00 am to 16.00 7 814 Himmatnagar Himmatnagar Sabar Kantha Gujarat Godown, Nr. Civil Circle, Station Road, pm Himmatnagar, Gujarat - 383001. Kotak Mahindra Bank Ltd., Kunal Complex,Opp. J.K. Petrol Pump,Highway 10.00 am to 16.00 8 815 Kadi Kadi Mehesana Gujarat Cross Roads, Kadi, Mehsana, Gujarat - pm 382715.