Microcystic Adnexal Carcinoma: an Immunohistochemical Reappraisal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Malignant Nodular Hidradenoma-Inguinal Region Clinically Masquerading As Squamous Cell Carcinoma: a Case Report
International Journal of Research in Medical Sciences Vernekar S et al. Int J Res Med Sci. 2019 Jul;7(7):2848-2852 www.msjonline.org pISSN 2320-6071 | eISSN 2320-6012 DOI: http://dx.doi.org/10.18203/2320-6012.ijrms20192933 Case Report Malignant nodular hidradenoma-inguinal region clinically masquerading as squamous cell carcinoma: a case report Sunita S. Vernekar, Priyadharshini Bargunam* Department of Pathology, Karnataka Institute of Medical Sciences, Hubballi, Karnataka, India Received: 24 April 2019 Accepted: 05 June 2019 *Correspondence: Dr. Priyadharshini Bargunam, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Malignant Nodular hidradenoma is an extremely rare aggressive tumour originating from eccrine sweat glands with an incidence of <.001%. So far less than 80 cases have been reported in the literature. It’s known for its local recurrence (50%) and metastasis (60%) and hence early diagnosis and radical treatment is mandatory. But differentiating it from its benign counterparts and other skin tumour mimics is challenging, due to its histopathological similarity & lack of diagnostic immunomarkers. Authors report a case of 65-year-old female who presented with a short 4-month history of rapidly growing ulceroproliferative growth in the right inguinal region with bilateral inguinal node enlargement, associated with pain and discharge. Wedge biopsy of left inguinal lymph node showed malignant cutaneous adnexal tumour deposits, which after excision was typed as malignant nodular hidradenoma. -

University of Dundee Hidradenoma Masquerading Digital
CORE Metadata, citation and similar papers at core.ac.uk Provided by University of Dundee Online Publications University of Dundee Hidradenoma masquerading digital ganglion cyst Makaram, Navnit; Chaudhry, Iskander H.; Srinivasan, Makaram S. Published in: Annals of Medicine and Surgery DOI: 10.1016/j.amsu.2016.07.017 Publication date: 2016 Document Version Publisher's PDF, also known as Version of record Link to publication in Discovery Research Portal Citation for published version (APA): Makaram, N., Chaudhry, I. H., & Srinivasan, M. S. (2016). Hidradenoma masquerading digital ganglion cyst: a rare phenomenon. Annals of Medicine and Surgery , 10, 22-26. DOI: 10.1016/j.amsu.2016.07.017 General rights Copyright and moral rights for the publications made accessible in Discovery Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from Discovery Research Portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain. • You may freely distribute the URL identifying the publication in the public portal. Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 17. Feb. 2017 Annals of Medicine and Surgery 10 (2016) 22e26 Contents lists available at ScienceDirect Annals of Medicine and Surgery journal homepage: www.annalsjournal.com Case report Hidradenoma masquerading digital ganglion cyst: A rare phenomenon * Navnit Makaram a, , Iskander H. -

Eyelid Conjunctival Tumors
EYELID &CONJUNCTIVAL TUMORS PHOTOGRAPHIC ATLAS Dr. Olivier Galatoire Dr. Christine Levy-Gabriel Dr. Mathieu Zmuda EYELID & CONJUNCTIVAL TUMORS 4 EYELID & CONJUNCTIVAL TUMORS Dear readers, All rights of translation, adaptation, or reproduction by any means are reserved in all countries. The reproduction or representation, in whole or in part and by any means, of any of the pages published in the present book without the prior written consent of the publisher, is prohibited and illegal and would constitute an infringement. Only reproductions strictly reserved for the private use of the copier and not intended for collective use, and short analyses and quotations justified by the illustrative or scientific nature of the work in which they are incorporated, are authorized (Law of March 11, 1957 art. 40 and 41 and Criminal Code art. 425). EYELID & CONJUNCTIVAL TUMORS EYELID & CONJUNCTIVAL TUMORS 5 6 EYELID & CONJUNCTIVAL TUMORS Foreword Dr. Serge Morax I am honored to introduce this Photographic Atlas of palpebral and conjunctival tumors,which is the culmination of the close collaboration between Drs. Olivier Galatoire and Mathieu Zmuda of the A. de Rothschild Ophthalmological Foundation and Dr. Christine Levy-Gabriel of the Curie Institute. The subject is now of unquestionable importance and evidently of great interest to Ophthalmologists, whether they are orbital- palpebral specialists or not. Indeed, errors or delays in the diagnosis of tumor pathologies are relatively common and the consequences can be serious in the case of malignant tumors, especially carcinomas. Swift diagnosis and anatomopathological confirmation will lead to a treatment, discussed in multidisciplinary team meetings, ranging from surgery to radiotherapy. -

Histopathology of Dermal Adnexal Tumours - a Four Years Study
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2013): 6.14 | Impact Factor (2015): 6.391 Histopathology of Dermal Adnexal Tumours - A Four Years Study Mukund Dhokiya1, Dr. Hemlata Talwelkar2, Dr. Sanjay Talwelkar3 13rd year Postgraduate Student, PDU Medical College & Hospital, Rajkot, India 2DA, MBBS, PDU Medical College & Hospital, Rajkot, India 3MD Pathology, Associate Professor, PDU Medical College & Hospital, Rajkot, India Abstract: Background: Dermal adnexal tumours (DAT) are a large and diverse group of benign and malignant tumours which exhibit morphological differentiation towards one of the different types of adnexal tumours present in normal skin: pilosebaeceous unit, eccrine and apocrine. Methods: 50 cases of skin adnexal tumours diagnosed in histopathological study over a period of 4 years (May 2012 to September 2016) in the Department of Pathology, PDU Medical College, Rajkot. Histopathological study is done in Formalin fixed, Paraffin embedded tissue sections stained with Haematoxylin and Eosin. Results: Skin adnexal tumours found most common in the age group of 21 to 60 years (74%, 37/50). Male to female ratio was 1:1.2. 98% cases found benign with only a single case (2%) malignant. The sweat gland tumors formed the largest group involving 52% of cases followed by hair follicle tumors (40%),sebaceous gland tumours (6%) and mixed (2%). Nodular hidradenoma (22%) and trichilemmal cyst (22%) found the most common benign tumours. Chondroid syringoma with malignant changes is the only malignant adnexal tumour reported in our study. Conclusion: Dermal adnexal tumours are relatively rare. Benign adnexal tumors are far more common than their malignant counterparts. -

Inherited Skin Tumour Syndromes
CME GENETICS Clinical Medicine 2017 Vol 17, No 6: 562–7 I n h e r i t e d s k i n t u m o u r s y n d r o m e s A u t h o r s : S a r a h B r o w n , A P a u l B r e n n a n B a n d N e i l R a j a n C This article provides an overview of selected genetic skin con- and upper trunk. 1,2 These lesions are fibrofolliculomas, ditions where multiple inherited cutaneous tumours are a cen- trichodiscomas and acrochordons. Patients are also susceptible tral feature. Skin tumours that arise from skin structures such to the development of renal cell carcinoma, lung cysts and as hair, sweat glands and sebaceous glands are called skin pneumothoraces. 3 appendage tumours. These tumours are uncommon, but can Fibrofolliculomas and trichodiscomas clinically present as ABSTRACT have important implications for patient care. Certain appenda- skin/yellow-white coloured dome shaped papules 2–4 mm in geal tumours, particularly when multiple lesions are seen, may diameter (Fig 1 a and Fig 1 b). 4 These lesions usually develop indicate an underlying genetic condition. These tumours may in the third or fourth decade.4 In the case of fibrofolliculoma, not display clinical features that allow a secure diagnosis to be hair specific differentiation is seen, whereas in the case of made, necessitating biopsy and dermatopathological assess- trichodiscoma, differentiation is to the mesodermal component ment. -

Cancer Immunoprevention: a Case Report Raising the Possibility of “Immuno-Interception” Jessica G
CANCER PREVENTION RESEARCH | RESEARCH BRIEF Cancer Immunoprevention: A Case Report Raising the Possibility of “Immuno-interception” Jessica G. Mancuso1, William D. Foulkes1,2,3, and Michael N. Pollak1,2 ABSTRACT ◥ Immune checkpoint blockade therapy provides substan- or neoplastic lesions over a period of 19 years (mean tial benefits for subsets of patients with advanced cancer, 7.5 neoplasms/year, range 2–26) prior to receiving but its utility for cancer prevention is unknown. Lynch pembrolizumab immunotherapy as part of multi- syndrome (MIM 120435) is characterized by defective modality treatment for invasive bladder cancer. He not DNA mismatch repair and predisposition to multiple only had a complete response of the bladder cancer, but cancers. A variant of Lynch syndrome, Muir–Torre also was noted to have an absence of new cancers during a syndrome (MIM 158320), is characterized by frequent 22-month follow-up period. This case adds to the rationale gastrointestinal tumors and hyperplastic or neoplastic skin for exploring the utility of immune checkpoint blockade tumors. We report the case of a man with Muir–Torre forcancerprevention,particularlyforpatientswithDNA syndrome who had 136 cutaneous or visceral hyperplastic repair deficits. Introduction There is an obvious clinical need to reduce cancer incidence in patients with DNA repair deficits, and prophylactic surgery The clinically demonstrated utility of antiviral vaccines to is commonly employed. Clinical trials designed to evaluate reduce risk of virally initiated cancers represents a major strategies to reduce cancer incidence are challenging: in popu- success in cancer immunoprevention. There is interest in the lations where baseline risk is low, a large number of subjects and possibility that immunoprevention may also be useful where long follow-up periods are required. -

2016 Essentials of Dermatopathology Slide Library Handout Book
2016 Essentials of Dermatopathology Slide Library Handout Book April 8-10, 2016 JW Marriott Houston Downtown Houston, TX USA CASE #01 -- SLIDE #01 Diagnosis: Nodular fasciitis Case Summary: 12 year old male with a rapidly growing temple mass. Present for 4 weeks. Nodular fasciitis is a self-limited pseudosarcomatous proliferation that may cause clinical alarm due to its rapid growth. It is most common in young adults but occurs across a wide age range. This lesion is typically 3-5 cm and composed of bland fibroblasts and myofibroblasts without significant cytologic atypia arranged in a loose storiform pattern with areas of extravasated red blood cells. Mitoses may be numerous, but atypical mitotic figures are absent. Nodular fasciitis is a benign process, and recurrence is very rare (1%). Recent work has shown that the MYH9-USP6 gene fusion is present in approximately 90% of cases, and molecular techniques to show USP6 gene rearrangement may be a helpful ancillary tool in difficult cases or on small biopsy samples. Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors, 5th edition. Mosby Elsevier. 2008. Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, Ye Y, Lau AW, Wang X, Oliveira AM. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011 Oct;91(10):1427-33. Amary MF, Ye H, Berisha F, Tirabosco R, Presneau N, Flanagan AM. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013 Jul;463(1):97-8. CONTRIBUTED BY KAREN FRITCHIE, MD 1 CASE #02 -- SLIDE #02 Diagnosis: Cellular fibrous histiocytoma Case Summary: 12 year old female with wrist mass. -

Genetics of Skin Appendage Neoplasms and Related Syndromes
811 J Med Genet: first published as 10.1136/jmg.2004.025577 on 4 November 2005. Downloaded from REVIEW Genetics of skin appendage neoplasms and related syndromes D A Lee, M E Grossman, P Schneiderman, J T Celebi ............................................................................................................................... J Med Genet 2005;42:811–819. doi: 10.1136/jmg.2004.025577 In the past decade the molecular basis of many inherited tumours in various organ systems such as the breast, thyroid, and endometrium.2 syndromes has been unravelled. This article reviews the clinical and genetic aspects of inherited syndromes that are Clinical features of Cowden syndrome characterised by skin appendage neoplasms, including The cutaneous findings of Cowden syndrome Cowden syndrome, Birt–Hogg–Dube syndrome, naevoid include trichilemmomas, oral papillomas, and acral and palmoplantar keratoses. The cutaneous basal cell carcinoma syndrome, generalised basaloid hallmark of the disease is multiple trichilemmo- follicular hamartoma syndrome, Bazex syndrome, Brooke– mas which present clinically as rough hyperker- Spiegler syndrome, familial cylindromatosis, multiple atotic papules typically localised on the face (nasolabial folds, nose, upper lip, forehead, ears3 familial trichoepitheliomas, and Muir–Torre syndrome. (fig 1A, 1C, 1D). Trichilemmomas are benign ........................................................................... skin appendage tumours or hamartomas that show differentiation towards the hair follicles kin consists of both epidermal and dermal (specifically for the infundibulum of the hair 4 components. The epidermis is a stratified follicle). Oral papillomas clinically give the lips, Ssquamous epithelium that rests on top of a gingiva, and tongue a ‘‘cobblestone’’ appearance basement membrane, which separates it and its and histopathologically show features of 3 appendages from the underlying mesenchymally fibroma. The mucocutaneous manifestations of derived dermis. -

Sebaceous Cell Carcinoma: a Persistent Challenge in Clinical And
erimenta xp l D E e r & m l a a t c o i l n o i Journal of Clinical & Experimental l g y C f R o e l ISSN: 2155-9554 s a e n Miyamoto et al., J Clin Exp Dermatol Res 2016, 7:3 a r r u c o h J Dermatology Research DOI: 10.4172/2155-9554.1000353 Review Article Open Access Sebaceous Cell Carcinoma: A Persistent Challenge in Clinical and Histopathological Diagnosis Denise Miyamoto1*, Beatrice Wang2, Cristina Miyamoto3,4, Valeria Aoki1, Li Anne Lim4, Paula Blanco4 and Miguel N Burnier4 1Department of Dermatology, University of São Paulo Medical School, Brazil 2Department of Dermatology, McGill University, Canada 3Department of Ophthalmology, Federal University of São Paulo, Brazil 4From the Henry C. Witelson Ocular Pathology Laboratory, McGill University, Canada *Corresponding author: Denise Miyamoto, University of São Paulo Medical School, São Paulo-State of São Paulo, Brazil, Tel: 514-934-76129; E-mail: [email protected] Received date: April 25, 2016; Accepted date: May 20, 2016; Published date: May 25, 2016 Copyright: © 2016 Miyamoto D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Sebaceous cell carcinoma continues to defy clinicians and pathologists in terms of early diagnosis. The tumor may be mistaken as benign lesions such as chalazion and blepharitis, and also as malignant neoplasms, mainly basal cell carcinoma and squamous cell carcinoma. Despite advances in immunohistochemical analysis and treatment options during the last decades, morbidity and metastasis rates remain high. -
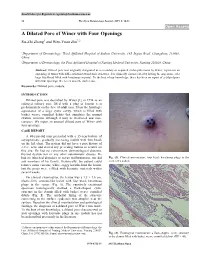
A Dilated Pore of Winer with Four Openings Ru-Zhi Zhang1 and Wen-Yuan Zhu*,2
Send Orders for Reprints to [email protected] 10 The Open Dermatology Journal, 2015, 9, 10-11 Open Access A Dilated Pore of Winer with Four Openings Ru-Zhi Zhang1 and Wen-Yuan Zhu*,2 1Department of Dermatology, Third Affiliated Hospital of Suzhou University, 185 Juqian Road, Changzhou, 213003, China 2Department of Dermatology, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, China Abstract: Dilated pore was originally designated as a secondary or acquired trichoepithelioma by Winer, represents an appendageal tumor with differentiation towards hair structures. It is clinically characterized by having the appearance of a large blackhead filled with keratinous material. To the best of our knowledge, there has been no report of a dilated pore with four openings. We herein describe such a case. Keywords: Dilated pore, nodule. INTRODUCTION Dilated pore was described by Winer [1] in 1954 as an enlarged solitary pore filled with a plug of keratin seen predominately on the face of adult men. It has the histologic appearance of a large cystic cavity, which is filled with basket weave cornified debris that simulates the normal stratum corneum although it may be thickened and more compact. We report an unusual dilated pore of Winer with four openings. CASE REPORT A 68-year-old man presented with a 35-year-history of asymptomatic, gradually increasing nodule with four heads on the left chest. The patient did not have a past history of severe acne and denied any preceding trauma or scratch on this site. He had no concomitant dermatological diseases, thyroid dysfunction or any other autoimmune disease. -

A Study of Cutaneous Adnexal Lesions-A Two Year Institutional Study
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 17, Issue 01 Ver. VI January. (2018), PP 16-23 www.iosrjournals.org A Study of Cutaneous Adnexal Lesions-A Two Year Institutional Study *Dr. K. Valarmathi1, Dr.Nalli.R.Sumitra Devi2,,Dr.P.Arunalatha3, Dr.Mrinalini 4 1,2,3,4,Department of Pathology,Govt.Stanley Medical College,The Tamil Nadu Dr.M.G.R. Medical University, Chennai, India) Corresponging Author: Dr. Nalli.R.Sumitra Devi Abstract Introduction: Tumors arising from the skin comprises a gamut of benign and malignant cutaneous lesions.Histopathology study of these lesions is quiet challenging due to its varied presentation. Aim:This cross sectional descriptive study is focussed to analyse the various cutaneous adnexal tumors with regard to age,sex,site,size, behaviour, origin of these tumors and to correlate with the clinical presentation. Methodology: 100 cutaneous adnexal lesions reported in the Department of Pathology,Stanley Medical College over a period of 2 years were taken up for the study. Sections were stained with routine haematoxylin and eosin stains followed by histopathological examination. Results: Out of the 100 cases 97 were benign and 3 were of malignant skin adnexal tumors. Sweat gland tumors were the most common tumors encountered and accounted to 51%. Conclusion:Though skin adnexal tumors are a rarity we recorded 97 benign and 3 cases of malignant adnexal tumors.Histopathological evaluation is of great value in arriving at a diagnosis of such lesions thereby aiding appropriate management . Keywords: Histomorphology, , hair follicular differentiation, skin adnexal tumors --------------------------------------------------------------------------------------------------------------------------------------- Date of Submission: 222-12-2017 Date of acceptance:03-01-2018 ----------------------------------------------------------------------------------------------------------------------------- ---------- I. -

Malignant Eccrine Acrospiroma with Nodal and Bone Metastasis
Case Report Malignant eccrine acrospiroma with nodal and bone metastasis Burhan Wani, Shiekh Aejaz Aziz, Mohmad Hussain Mir, Gull Mohammad Bhat, Abdul Rashid Lone Department of Medical Oncology, Sher-i-Kashmir Institute of Medical Sciences, Srinagar 190011, Kashmir, India. Correspondence to: Dr. Burhan Wani, Department of Medical Oncology, Sher-i-Kashmir Institute of Medical Sciences, Srinagar 190011, Kashmir, India. E-mail: [email protected] ABSTRACT Acrospiromas are cutaneous tumors of sweat duct differentiation. Although various eccrine sweat gland tumours including benign acrospiroma are widely reviewed, malignant acrospiroma is rarely reported. Clinically, they resemble other cutaneous lesions and the primary treatment is wide local excision with or without lymph node dissection. The efficacy of adjuvant chemotherapy and radiation therapy requires further investigation. Key words: Acrospiroma; metastasis; chemotherapy; radiotherapy INTRODUCTION right inguinal region. The swelling was firm in consistency and mildly tender. There was another mass 2 cm below Acrospiroma represents a group of benign ductal tumors this measuring 3 cm × 2 cm, firm in consistency, mobile, of the eccrine sweat glands that sometimes are connected non-tender with normal overlying skin, felt to be a lymph to the skin, ranging from solitary plaques to exophytic node clinically. papules or dermal nodules.[1] Malignant acrospiroma (Syn: malignant nodular/clear cell hidradenoma, malignant clear The patient was operated on and excision of the mass cell acrospiroma, clear cell eccrine carcinoma, primary along with inguinal nodal dissection. Pathology revealed mucoepidermoid cutaneous carcinoma) comprises a dermal appendage neoplasm (acrospiroma -- of hydra group of rare epidermal, juxta-epidermal, and dermal adenoma type), well-circumscribed, with mitotic figures ductal carcinomas that may coexist with their benign (< 2/hpf).