Oxidation State of Organic Molecules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Redox Models in Chemistry
Redox models in chemistry A depiction of the conceptions held by secondary school students of redox reactions Lise-Lotte Österlund Department of Chemistry 901 87 Umeå Umeå 2010 Copyright©Lise-Lotte Österlund ISBN: 978-91-7459-053-1 ISSN: 1652-5051 Cover picture: Photographer, Lise-Lotte Österlund Printed by: VMC, KBC, Umeå University Umeå, Sweden 2010 T0 my family Abstract According to previous research, students show difficulties in learning redox reactions. By the historical development different redox models exist to explain redox reactions, the oxygen model, the hydrogen model, the electron model and the oxidation number model. This thesis reports about three studies concerning conceptions held by secondary school students of redox reactions. A textbook analysis is also included in the thesis. The first study was an investigation of the students’ use of redox models in inorganic contexts, their use of the activity series of metals, and the students’ ability to transfer redox knowledge. Then the students’ work with an open- ended biochemical task, where the students had access of the textbook was studied. The students talk about redox reactions, the questions raised by the students, what resources used to answer the questions and what kind of talk developed were investigated. A textbook analysis based on chemistry books from Sweden and one book from England was performed. The redox models used as well as the dealing with redox related learning difficulties was studied. Finally, the students’ conceptions about redox in inorganic, organic and biochemistry after completed chemistry courses were studied. The results show that the students were able to use the electron model as a tool to explain inorganic redox reactions and the mutuality of oxidation and reduction was fundamental. -

Promotion Effects and Mechanism of Alkali Metals and Alkaline Earth
Subscriber access provided by RES CENTER OF ECO ENVIR SCI Article Promotion Effects and Mechanism of Alkali Metals and Alkaline Earth Metals on Cobalt#Cerium Composite Oxide Catalysts for N2O Decomposition Li Xue, Hong He, Chang Liu, Changbin Zhang, and Bo Zhang Environ. Sci. Technol., 2009, 43 (3), 890-895 • DOI: 10.1021/es801867y • Publication Date (Web): 05 January 2009 Downloaded from http://pubs.acs.org on January 31, 2009 More About This Article Additional resources and features associated with this article are available within the HTML version: • Supporting Information • Access to high resolution figures • Links to articles and content related to this article • Copyright permission to reproduce figures and/or text from this article Environmental Science & Technology is published by the American Chemical Society. 1155 Sixteenth Street N.W., Washington, DC 20036 Environ. Sci. Technol. 2009, 43, 890–895 Promotion Effects and Mechanism such as Fe-ZSM-5 are more active in the selective catalytic reduction (SCR) of N2O by hydrocarbons than in the - ° of Alkali Metals and Alkaline Earth decomposition of N2O in a temperature range of 300 400 C (3). In recent years, it has been found that various mixed Metals on Cobalt-Cerium oxide catalysts, such as calcined hydrotalcite and spinel oxide, showed relatively high activities. Composite Oxide Catalysts for N2O One of the most active oxide catalysts is a mixed oxide containing cobalt spinel. Calcined hydrotalcites containing Decomposition cobalt, such as Co-Al-HT (9-12) and Co-Rh-Al-HT (9, 11), have been reported to be very efficient for the decomposition 2+ LI XUE, HONG HE,* CHANG LIU, of N2O. -

Prebiological Evolution and the Metabolic Origins of Life
Prebiological Evolution and the Andrew J. Pratt* Metabolic Origins of Life University of Canterbury Keywords Abiogenesis, origin of life, metabolism, hydrothermal, iron Abstract The chemoton model of cells posits three subsystems: metabolism, compartmentalization, and information. A specific model for the prebiological evolution of a reproducing system with rudimentary versions of these three interdependent subsystems is presented. This is based on the initial emergence and reproduction of autocatalytic networks in hydrothermal microcompartments containing iron sulfide. The driving force for life was catalysis of the dissipation of the intrinsic redox gradient of the planet. The codependence of life on iron and phosphate provides chemical constraints on the ordering of prebiological evolution. The initial protometabolism was based on positive feedback loops associated with in situ carbon fixation in which the initial protometabolites modified the catalytic capacity and mobility of metal-based catalysts, especially iron-sulfur centers. A number of selection mechanisms, including catalytic efficiency and specificity, hydrolytic stability, and selective solubilization, are proposed as key determinants for autocatalytic reproduction exploited in protometabolic evolution. This evolutionary process led from autocatalytic networks within preexisting compartments to discrete, reproducing, mobile vesicular protocells with the capacity to use soluble sugar phosphates and hence the opportunity to develop nucleic acids. Fidelity of information transfer in the reproduction of these increasingly complex autocatalytic networks is a key selection pressure in prebiological evolution that eventually leads to the selection of nucleic acids as a digital information subsystem and hence the emergence of fully functional chemotons capable of Darwinian evolution. 1 Introduction: Chemoton Subsystems and Evolutionary Pathways Living cells are autocatalytic entities that harness redox energy via the selective catalysis of biochemical transformations. -

Redox Potential Measurements M.J
Redox Potential Measurements M.J. Vepraskas NC State University December 2002 Redox potential is an electrical measurement that shows the tendency of a soil solution to transfer electrons to or from a reference electrode. From this measurement we can estimate whether the soil is aerobic, anaerobic, and whether chemical compounds such as Fe oxides or nitrate have been chemically reduced or are present in their oxidized forms. Making these measurements requires three basic pieces of equipment: 1. Platinum electrode 2. Voltmeter 3. Reference electrode The basic set-up is shown below: Voltmeter 456 mv Soil surface Reference Pt wire electrode Fig. 1. The Pt wire is buried into the soil to be in contact with the soil solution. The reference electrode must also be in contact with the soil solution. It has a ceramic tip, which can be placed in the soil, or the electrode can be placed in a salt bridge, which is itself placed in the soil. Wires from both the Pt electrode and reference electrode are connected to the voltmeter. Pt Electrodes Platinum electrodes consist of a small piece of platinum wire that is soldered or fused to wire made another metal. Platinum conducts electrons from the soil solution to the wire to which it is attached. Platinum is used because it is assumed to be an inert metal. This means it does not give up its own electrons (does not oxidize) to the wire or soil solution. Iron containing materials such as steel will oxidize themselves and send their own electrons to the voltmeter. As a result the voltage we measure will not result solely from electrons being transferred to or from the soil. -

Oxidation State Slides.Key
Oxidation States Dr. Sobers’ Lecture Slides The Oxidation State Also known as the oxidation number The oxidation state is used to determine whether an element has been oxidized or reduced. The oxidation state is not always a real, quantitative, physical constant. The oxidation state can be the charge on an atom: 2+ - MgCl2 Mg Cl Oxidation State: +2 -1 2 The Oxidation State For covalently bonded substances, it is not as simple as an ionic charge. A covalent bond is a sharing of electrons. The electrons are associated with more than one atomic nuclei. This holds the nuclei together. The electrons may not be equally shared. This creates a polar bond. The electronegativity of a covalently bonded atom is its ability to attract electrons towards itself. 3 Example: Chlorine Sodium chloride is an ionic compound. In sodium chloride, the chloride ion has a charge and an oxidation state of -1. The oxidation state of sodium is +1. 4 Example: Chlorine In a chlorine molecule, the chlorine atoms are covalently bonded. The two atoms share electrons equally and the oxidation state is 0. 5 Example: Chlorine The two atoms of a hydrogen chloride molecule are covalently bonded. The electrons are not shared equally because chlorine is more electronegative than hydrogen. There are no ions but the oxidation state of chlorine in HCl is -1 and the oxidation state of hydrogen is +1. 6 7 Assigning Oxidation States See the handout for the list of rules. Rule 1: Free Elements Free elements have an oxidation state of zero Example Oxidation State O2(g) 0 Fe(s) 0 O3(g) 0 C(graphite) 0 C(diamond) 0 9 Rule 2: Monatomic Ions The oxidation state of monatomic ions is the charge of the ion Example Oxidation State O2- -2 Fe3+ +3 Na+ +1 I- -1 V4+ +4 10 Rule 3: Fluorine in Compounds Fluorine in a compound always has an oxidation state of -1 Example Comments and Oxidation States NaF These are monatomic ions. -

Origins of Life in the Universe Zackary Johnson
11/4/2007 Origins of Life in the Universe Zackary Johnson OCN201 Fall 2007 [email protected] Zackary Johnson http://www.soest.hawaii.edu/oceanography/zij/education.html Uniiiversity of Hawaii Department of Oceanography Class Schedule Nov‐2Originsof Life and the Universe Nov‐5 Classification of Life Nov‐7 Primary Production Nov‐9Consumers Nov‐14 Evolution: Processes (Steward) Nov‐16 Evolution: Adaptation() (Steward) Nov‐19 Marine Microbiology Nov‐21 Benthic Communities Nov‐26 Whale Falls (Smith) Nov‐28 The Marine Food Web Nov‐30 Community Ecology Dec‐3 Fisheries Dec‐5Global Ecology Dec‐12 Final Major Concepts TIMETABLE Big Bang! • Life started early, but not at the beginning, of Earth’s Milky Way (and other galaxies formed) history • Abiogenesis is the leading hypothesis to explain the beginning of life on Earth • There are many competing theories as to how this happened • Some of the details have been worked out, but most Formation of Earth have not • Abiogenesis almost certainly occurred in the ocean 20‐15 15‐94.5Today Billions of Years Before Present 1 11/4/2007 Building Blocks TIMETABLE Big Bang! • Universe is mostly hydrogen (H) and helium (He); for Milky Way (and other galaxies formed) example –the sun is 70% H, 28% He and 2% all else! Abundance) e • Most elements of interest to biology (C, N, P, O, etc.) were (Relativ 10 produced via nuclear fusion Formation of Earth Log at very high temperature reactions in large stars after Big Bang 20‐13 13‐94.7Today Atomic Number Billions of Years Before Present ORIGIN OF LIFE ON EARTH Abiogenesis: 3 stages Divine Creation 1. -

The Structural and Chemical Origin of the Oxygen Redox Activity in Layered and Cation-Disordered Li-Excess Cathode Materials
ARTICLES PUBLISHED ONLINE: 30 MAY 2016 | DOI: 10.1038/NCHEM.2524 The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials Dong-Hwa Seo1,2†, Jinhyuk Lee1,2†, Alexander Urban2, Rahul Malik1,ShinYoungKang1 and Gerbrand Ceder1,2,3* Lithium-ion batteries are now reaching the energy density limits set by their electrode materials, requiring new paradigms for Li+ and electron hosting in solid-state electrodes. Reversible oxygen redox in the solid state in particular has the potential to enable high energy density as it can deliver excess capacity beyond the theoretical transition-metal redox- capacity at a high voltage. Nevertheless, the structural and chemical origin of the process is not understood, preventing the rational design of better cathode materials. Here, we demonstrate how very specific local Li-excess environments around oxygen atoms necessarily lead to labile oxygen electrons that can be more easily extracted and participate in the practical capacity of cathodes. The identification of the local structural components that create oxygen redox sets a new direction for the design of high-energy-density cathode materials. ver the past two decades, lithium-ion battery technology has why it is observed with some metals and not with others. The contributed greatly to human progress and enabled many of specific nature of our findings reveals a clear and exciting path Othe conveniences of modern life by powering increasingly towards creating the next generation of cathode materials with capable portable electronics1. However, with the increasing com- substantially higher energy density than current cathode materials. -

Structure-Property Relationships of Layered Oxypnictides
AN ABSTRACT OF THE DISSERTATION OF Sean W. Muir for the degree of Doctor of Philosophy in Chemistry presented on April 17, 2012. Title: Structure-property Relationships of Layered Oxypnictides Abstract approved:______________________________________________________ M. A. Subramanian Investigating the structure-property relationships of solid state materials can help improve many of the materials we use each day in life. It can also lead to the discovery of materials with interesting and unforeseen properties. In this work the structure property relationships of newly discovered layered oxypnictide phases are presented and discussed. There has generally been worldwide interest in layered oxypnictide materials following the discovery of superconductivity up to 55 K for iron arsenides such as LnFeAsO1-xFx (where Ln = Lanthanoid). This work presents efforts to understand the structure and physical property changes which occur to LnFeAsO materials when Fe is replaced with Rh or Ir and when As is replaced with Sb. As part of this work the solid solution between LaFeAsO and LaRhAsO was examined and superconductivity is observed for low Rh content with a maximum critical temperature of 16 K. LnRhAsO and LnIrAsO compositions are found to be metallic; however Ce based compositions display a resistivity temperature dependence which is typical of Kondo lattice materials. At low temperatures a sudden drop in resistivity occurs for both CeRhAsO and CeIrAsO compositions and this drop coincides with an antiferromagnetic transition. The Kondo scattering temperatures and magnetic transition temperatures observed for these materials can be rationalized by considering the expected difference in N(EF)J parameters between them, where N(EF) is the density of states at the Fermi level and J represents the exchange interaction between the Ce 4f1 electrons and the conduction electrons. -

Thermochemical Energy Storage by Water-Splitting Via Redox Reaction of Alkali Metals
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 49 ( 2014 ) 927 – 934 SolarPACES 2013 Thermochemical energy storage by water-splitting via redox reaction of alkali metals H. Miyaokaa,*, T. Ichikawab, Y. Kojimab aInstitute for Sustainable Sciences and Development, Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima, Hiroshima, 739-8530, Japan bInstitute for Advanced Materials Research, Hiroshima University1-3-1 Kagamiyama, Higashi-Hiroshima, Hiroshima, 739-8530, Japan Abstract The reaction cycles for water-splitting based on redox reactions of alkali metals are composed of four reactions, which are hydrogen generation by solid-liquid reaction, metal separation by thermolysis, oxygen generation by hydrolysis, and phase transition of the metal. Although all the cycles theoretically require more than 1000 qC in thermodynamic equilibrium condition, the reaction temperature are reduced to below 800 qCn− by no equilibrium techniques using phase transition of metal vapor. In this work, thermodynamic analyses are performed by using the parameters such as operating temperature and partial pressures of the products obtained by the experiments to determine that the alkali metal redox cycles are potential hydrogen production technique as thermochemical energy storage. © 2013 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (©http://creativecommons.org/licenses/by-nc-nd/3.0/ 2013 The Authors. Published by Elsevier Ltd.). Selection and and peer peer review review by by the the scientific scientific conference conference committee committee of SolarPACES of SolarPACES 2013 under 2013 responsibility under responsibility of PSE AG. of PSE AG. Final manuscript published as received without editorial corrections. -
Plutonium Is a Physicist's Dream but an Engineer's Nightmare. with Little
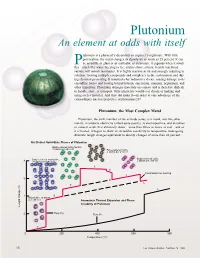
Plutonium An element at odds with itself lutonium is a physicist’s dream but an engineer’s nightmare. With little provocation, the metal changes its density by as much as 25 percent. It can Pbe as brittle as glass or as malleable as aluminum; it expands when it solidi- fies—much like water freezing to ice; and its shiny, silvery, freshly machined surface will tarnish in minutes. It is highly reactive in air and strongly reducing in solution, forming multiple compounds and complexes in the environment and dur- ing chemical processing. It transmutes by radioactive decay, causing damage to its crystalline lattice and leaving behind helium, americium, uranium, neptunium, and other impurities. Plutonium damages materials on contact and is therefore difficult to handle, store, or transport. Only physicists would ever dream of making and using such a material. And they did make it—in order to take advantage of the extraordinary nuclear properties of plutonium-239. Plutonium, the Most Complex Metal Plutonium, the sixth member of the actinide series, is a metal, and like other metals, it conducts electricity (albeit quite poorly), is electropositive, and dissolves in mineral acids. It is extremely dense—more than twice as dense as iron—and as it is heated, it begins to show its incredible sensitivity to temperature, undergoing dramatic length changes equivalent to density changes of more than 20 percent. Six Distinct Solid-State Phases of Plutonium Body-centered orthorhombic, 8 atoms per unit cell Face-centered cubic, 4 atoms per unit cell Body-centered monoclinic, Body-centered cubic, 8 34 atoms per unit cell 2 atoms per unit cell δ δ′ ε Contraction on melting 6 γ L L 4 β Monoclinic, 16 atoms per unit cell Anomalous Thermal Expansion and Phase Instability of Plutonium Length change (%) 2 Pure Pu Pure Al α 0 200 400 600 800 Temperature (°C) 16 Los Alamos Science Number 26 2000 Plutonium Overview Table I. -

Reduction of Nitrogen Oxides by Carbon Monoxide Over an Iron Oxide Catalyst Under Dynamic Conditions
Applied Catalysis B: Environmental 17 (1998) 357±369 Reduction of nitrogen oxides by carbon monoxide over an iron oxide catalyst under dynamic conditions Harvey Randall, Ralf Doepper, Albert Renken* Institute of Chemical Engineering, Federal Institute of Technology, 1015 Lausanne, Switzerland Received 6 July 1997; received in revised form 23 November 1997; accepted 2 December 1997 Abstract The reduction of NO and N2O by CO over a silica-supported iron oxide catalyst was investigated by the transient response method, with different initial oxidation states of the catalyst, i.e. completely reduced (Fe3O4), or oxidised (Fe2O3). The in¯uence of CO pre-adsorption was also studied. From the material balance on the gas phase species, it was shown that the composition of the catalyst changes during relaxation to steady-state. The degree of reduction of the catalyst at steady-state could thus be estimated. During the transient period, CO was shown to inhibit N2O as well as NO reductions by adsorption on reduced sites. The activity of the reduced catalyst was found to be substantially higher as compared to the oxidised catalyst for both reactions. On this basis, it was attempted to keep the catalyst in a reduced state by periodically reducing it with CO. As a result, a signi®cant increase in the performance of the reactor with respect to steady-state operation could be achieved for N2O reduction by CO. Finally, the dynamic behaviour of the N2O±CO and NO±CO reactions made it possible to evidence reaction steps, the occurrence of which could not be shown during our previous investigations on the separate interactions of the reactants with the catalyst. -

Chemical Redox Agents for Organometallic Chemistry
Chem. Rev. 1996, 96, 877−910 877 Chemical Redox Agents for Organometallic Chemistry Neil G. Connelly*,† and William E. Geiger*,‡ School of Chemistry, University of Bristol, U.K., and Department of Chemistry, University of Vermont, Burlington, Vermont 05405-0125 Received October 3, 1995 (Revised Manuscript Received January 9, 1996) Contents I. Introduction 877 A. Scope of the Review 877 B. Benefits of Redox Agents: Comparison with 878 Electrochemical Methods 1. Advantages of Chemical Redox Agents 878 2. Disadvantages of Chemical Redox Agents 879 C. Potentials in Nonaqueous Solvents 879 D. Reversible vs Irreversible ET Reagents 879 E. Categorization of Reagent Strength 881 II. Oxidants 881 A. Inorganic 881 1. Metal and Metal Complex Oxidants 881 2. Main Group Oxidants 887 B. Organic 891 The authors (Bill Geiger, left; Neil Connelly, right) have been at the forefront of organometallic electrochemistry for more than 20 years and have had 1. Radical Cations 891 a long-standing and fruitful collaboration. 2. Carbocations 893 3. Cyanocarbons and Related Electron-Rich 894 Neil Connelly took his B.Sc. (1966) and Ph.D. (1969, under the direction Compounds of Jon McCleverty) degrees at the University of Sheffield, U.K. Post- 4. Quinones 895 doctoral work at the Universities of Wisconsin (with Lawrence F. Dahl) 5. Other Organic Oxidants 896 and Cambridge (with Brian Johnson and Jack Lewis) was followed by an appointment at the University of Bristol (Lectureship, 1971; D.Sc. degree, III. Reductants 896 1973; Readership 1975). His research interests are centered on synthetic A. Inorganic 896 and structural studies of redox-active organometallic and coordination 1.