Vitamin a and Retinoid Derivatives for Lung Cancer: a Systematic Review and Meta Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vitamin A: History, Current Uses, and Controversies M
Vitamin A: History, Current Uses, and Controversies M. Shane Chapman, MD Vitamin A is required for the proper functioning of many important metabolic and physio- logic activities, including vision, gene transcription, the immune system and skin cell differentiation. Both excessive and deficient levels of vitamin A lead to poor functioning of many human systems. The biologically active form, retinoic acid, binds to nuclear receptors that facilitate transcription that ultimately leads to it’s physiological effects. Retinoids are derivatives of vitamin A that are medications used to treat acne vulgaris, psoriasis, ichthyosis (and other disorders of keratinization), skin cancer prevention as well as several bone marrow derived neoplasias. Systemic retinoids are teratogenic and have to be prescribed with caution and close oversight. Other potential adverse events are contro- versial. These include the relationship of retinoid derivatives in sunscreens, their effects on bone mineral density, depression and suicidal ideation and inflammatory bowel disease. These controversies will be discussed in detail. Semin Cutan Med Surg 31:11-16 © 2012 Published by Elsevier Inc. KEYWORDS vitamin A, retinoids, carotenoids, sunscreens, bone metabolism, teratogenicity, depression, suicidal ideation, istotretinoin, inflammatory bowel disease. itamin A is a fat-soluble vitamin that is required for the molecules, retinal, for both low-light- and color vision. Ret- Vproper functioning of a diverse array of metabolic and inol is also converted to retinoic acid, which is a hormone- physiologic activities. Vision, hematopoiesis, embryonic de- like growth factor important for epithelial cell growth and velopment, skin cell differentiation, immune system func- differentiation. It is required for skin and bone health, but in tion, and gene transcription all require vitamin A. -
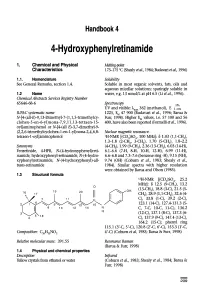
4-Hydroxyphenyiretinamide
Handbook 4 4-Hydroxyphenyiretinamide 1. Chemical and Physical Melting-point Characteristics 173-175 °C (Shealy etal., 1984; Budavari etal., 1996) 1.1. Nomenclature Solubility See General Remarks, section 1.4. Soluble in most organic solvents, fats, oils and aqueous micellar solutions; sparingly soluble in 1.2 Name water, e.g. 13 nmol/L at pH 6.5 (Li et al., 1996). Chemical Abstracts Services Registry Number 65646-68-6 Spectroscopy 1% UV and visible: ? 362 (methanol), E 1 IUPAC systematic name 1225, EM 47 900 (Budavari et al., 1996; Barua & N-[4-(all-E)-9, 13-Dimethyl-7-(1, 1,5-trimethylcy- Fun, 1998). Higher EM values, i.e. 57 100 and 56 clohex-5 -en-6-yl)nona- 7,9,11,1 3-tetraen- 15- 400, have also been reported (Formelli et al., 1996). oyl]aminophenol or N-[4-(all E)-3, 7-dimethyl-9- (2,2,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8- Nuclear magnetic resonance: tetraen-1-oyl]aminophenol 'H-NMR [(CD3)SO4, 100 MHz]: 8 1.03 (1,1-CH3), 1.3-1.8 (2-CH2, 3-CH), 1.70 (5-CH), 1.8-2.2 Synonyms (4-CH2), 1.99 (9-CH), 2.36 (13-CH), 6.03 (14-H), Fenretinide, 4-HPR, N-(4-hydroxyphenyl)reti- 6.1-6.6 (7-H, 8-H, 10-H, 12-H), 6.99 (11-H), namide, hydroxyphenyl-retinamide, N-(4-hydro- 6.6-6.8 and 7.3-7.6 (benzene ring -H), 9.15 (NH), xyphenyl)retinamide, N-(4-hydroxyphenyl)-all- 9.74 (OH) (Coburn et al., 1983; Shealy et al., tnins-retinamide 1984). -

Microencapsulation of Amorphous Solid Dispersions of Fenretinide Enhances Drug Solubility and Release from PLGA in Vitro and in Vivo T ⁎ Kari Nietoa, Susan R
International Journal of Pharmaceutics 586 (2020) 119475 Contents lists available at ScienceDirect International Journal of Pharmaceutics journal homepage: www.elsevier.com/locate/ijpharm Microencapsulation of amorphous solid dispersions of fenretinide enhances drug solubility and release from PLGA in vitro and in vivo T ⁎ Kari Nietoa, Susan R. Malleryb, Steven P. Schwendemana,c, a Department of Pharmaceutical Sciences and The Biointerfaces Institute, University of Michigan, Ann Arbor, MI, United States b Division of Oral Maxillofacial Pathology & Radiology, College of Dentistry, Ohio State University, Columbus, OH, United States c Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States ARTICLE INFO ABSTRACT Keywords: The purpose of this study was to develop solid dispersions of fenretinide(4HPR), incorporate them into poly Fenretinide (4HPR) (lactic-co-glycolic)(PLGA) millicylindrical implants, and evaluate the resulting implants in vitro and in vivo for polyvinylpyrrolidone (PVP) future applications in oral cancer chemoprevention. Due to the extreme hydrophobicity of 4HPR, 4HPR-poly- amorphous solid dispersion (ASD) vinylpyrrolidone (PVP) amorphous solid dispersions(ASDs) were prepared for solubility enhancement. The Solubility enhancement optimal PVP-4HPR ratio of 9/1(w/w) provided a 50-fold solubility enhancement in aqueous media, which was Poly(lactic-co-glycolic) (PLGA) sustained over 1 week. PVP-4HPR ASD particles were loaded into PLGA millicylinders and drug release was In vivo release long-acting release (LAR) evaluated in vitro in PBST and in vivo by recovery from subcutaneous injection in rats. While initial formulations of PLGA PVP-4HPR millicylinders only released 10% 4HPR in vitro after 28 days, addition of the plasticizer triethyl-o-acetyl-citrate(TEAC) into PVP-4HPR ASDs resulted in a 5.6-fold total increase in drug release. -

Agonist and Antagonist of Retinoic Acid Receptors Cause Similar Changes in Gene Expression and Induce Senescence-Like Growth Arrest in MCF-7 Breast Carcinoma Cells
Research Article Agonist and Antagonist of Retinoic Acid Receptors Cause Similar Changes in Gene Expression and Induce Senescence-like Growth Arrest in MCF-7 Breast Carcinoma Cells Yuhong Chen,1 Milos Dokmanovic,1 Wilfred D. Stein,1,2 Robert J. Ardecky,3 and Igor B. Roninson1 1Cancer Center, Ordway Research Institute, Albany, New York; 2Institute of Life Sciences, Hebrew University, Jerusalem, Israel; and 3Ligand Pharmaceuticals, Inc., San Diego, California Abstract retinoids is most often attributed to the induction of differentia- Biological effects of retinoids are mediated via retinoic acid tion, but these compounds were also shown to stop the growth of (RA) receptors (RAR) and retinoid X receptors (RXR). The tumor cells by inducing apoptosis or accelerated senescence (1, 2). best-characterized mechanism of retinoid action is stimula- In particular, treatment of two human breast carcinoma cell lines tion of transcription from promoters containing RA response with all-trans retinoic acid (RA) or fenretinide, in vitro or in vivo, elements (RARE). Retinoids induce senescence-like growth induces a senescence-like phenotype characterized by increased h arrest in MCF-7 breast carcinoma cells; this effect is cell size and expression of senescence-associated -galactosidase h associated with the induction of several growth-inhibitory (SA- -gal; refs. 3, 4). This phenotype, as investigated in MCF-7 cells, genes. We have nowfound that these genes are induced by is associated with irreversible growth arrest and up-regulation of RAR-specific but not by RXR-specific ligands. Genome-scale several intracellular and secreted proteins with known growth- microarray analysis of gene expression was used to compare inhibitory activities. -

Manual for Sugar Fortification with Vitamin a Part 3
Manual for Sugar Fortification with Vitamin A Part 3 Analytical Methods for the Control and Evaluation of Sugar Fortification with Vitamin A Omar Dary, Ph.D. Guillermo Arroyave, Ph.D. with Hernando Flores, Ph.D., Florisbela A. C. S. Campos, and Maria Helena C. B. Lins Dr. Omar Dary is a research biochemist at the Institute of Nutrition of Central America and Panama (INCAP), Guatemala. Dr. Guillermo Arroyave is an international consultant in micronutrients residing in San Diego, California. Dr. Hernando Flores, Ms. Campos, and Ms. Lins are biochemists at the Universidad de Pernambuco, Brazil. MANUAL FOR SUGAR FORTIFICATION PART 3 TABLE OF CONTENTS ACKNOWLEDGMENTS ........................................................... v FOREWORD ...................................................................vii I. INTRODUCTION .......................................................... 1 II. PROPERTIES OF RETINOL AND RETINOL COMPOUNDS USED IN SUGAR FORTIFICATION .......................................................... 3 III. PRINCIPLES FOR DETERMINING RETINOL IN VITAMIN A PREMIX AND FORTIFIED SUGAR .................................................................. 5 A. Spectrophotometric method ............................................. 5 B. Colorimetric method .................................................. 6 IV. SPECTROPHOTOMETRIC DETERMINATION OF RETINOL IN PREMIX ........... 7 A. References .......................................................... 7 B. Principle ............................................................ 7 C. Critical -

LC-MS-MS Quantitative Analysis of 12 Retinoids, Derivatives And
LC-MS-MS quantitative analysis of 12 Retinoids, derivatives and metabolites in serum for clinical research use Rory M Doyle, Joshua Kline, Thermo Scientific Inc., 265 Davidson Avenue, Somerset, NJ 08873 ABSTRACT Reagents Table 1- Scan Parameters- SRM table Figure 1: Chromatograms and Retention time The following Fisher Scientific™ acids, reagents and solvents were used- Compound Rt Polarity Precursor Product Collision Rf Introduction: Liquid chromatography triple quadrupole mass spectrometry is suited for rapid analysis (min) (m/z) (m/z) Energies Lens F:\ASMS-STD\100ngml-STD 05/25/17 19:11:02 100ngml-STD of multiple analytes of similar and different structures and physicochemical properties. Retinoids are HPLC grade Water Formic Acid (V) (V) a diverse group of three different generations of biologically active compounds that physiologically RT: 0.00 - 5.29 RT: 0.00 - 5.29 Methanol Acetonitile RT: 0.86 RT: 3.16 impact the bodies’ functions and include- retinol, retinal, tretinoin, isotretinoin, etretinate, acitretin, Tazarotenic Acid 0.86 Positive 324.2 294/308 34.3/36.4 191 AA: 17396997 AA: 116868 Methyl-Tert-Butyl-Ether (MTBE) 100 Tazarotenic Acid 100 Bexarotene adapalene, bexarotene, tazarotene and metabolites. A sensitive and specific LC-MS/MS analytical Tazarotene 1.67 Positive 352.2 324.01294 26.4/40.1 206 research method was developed and optimized for the quantitation of retinoids and metabolites in 0 0 RT: 1.67 RT: 3.27 serum. Simple sample preparation techniques were used that included a protein crash and liquid- The standards and internal standards were made up in Methanol. Acitretin 2.51 Positive 327.2 176.9/159 10.3/17.4 101 AA: 88457528 AA: 527912 100 Tazarotene 100 Retinoic Acid liquid extraction. -

The Mechanisms of Fenretinide-Mediated Anti-Cancer Activity and Prevention of Obesity and Type-2 Diabetes
*Manuscript Click here to view linked References The mechanisms of Fenretinide-mediated anti-cancer activity and 1 2 3 prevention of obesity and type-2 diabetes. 4 5 Authors: Nimesh Mody* and George D. Mcilroy. 6 7 8 Institute of Medical Sciences, College of Life Sciences & Medicine, University of Aberdeen, 9 10 Aberdeen, UK 11 12 * corresponding author 13 14 15 Running title: The synthetic retinoid, Fenretinide. 16 17 Abbreviations: Fenretinide, FEN; 18 19 20 Keywords: apoptosis, autophagy, reactive oxygen species, retinoic acid, adipogenesis, 21 22 obesity, type-2 diabetes. 23 24 25 Word count: 7169 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 1 63 64 65 ABSTRACT 1 2 3 4 Fenretinide remains the most investigated retinoid compound for the prevention of cancer. Its 5 6 clinical use remains a genuine possibility due to a favourable toxicological profile and 7 8 accumulation in fatty tissues. Like other well-characterised pharmacological therapies, 9 10 11 Fenretinide has been shown to affect multiple signalling pathways. Recent findings have 12 13 discovered additional beneficial properties the synthetic retinoid was not intentionally 14 15 16 designed for, including the prevention of high-fat diet-induced obesity and insulin resistance. 17 18 These preclinical findings in rodents are timely since obesity has reached pandemic 19 20 21 proportions and safe effective therapeutics are severely lacking. Recent investigations have 22 23 proposed various mechanisms of action for the beneficial effects of Fenretinide. -

A Group of Chimeric Antigen Receptors (Cars)
(19) *EP003632461A1* (11) EP 3 632 461 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.04.2020 Bulletin 2020/15 A61K 39/00 (2006.01) (21) Application number: 18198850.2 (22) Date of filing: 05.10.2018 (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • SALZER, Benjamin GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO 3744 Stockern (AT) PL PT RO RS SE SI SK SM TR •LEHNER, Manfred Designated Extension States: 1090 Vienna (AT) BA ME • TRAXLMAYR, Michael Designated Validation States: 1180 Vienna (AT) KH MA MD TN (74) Representative: Sonn & Partner Patentanwälte (71) Applicants: Riemergasse 14 • St. Anna Kinderkrebsforschung 1010 Wien (AT) 1090 Vienna (AT) • Universität für Bodenkultur Wien 1180 Wien (AT) (54) A GROUP OF CHIMERIC ANTIGEN RECEPTORS (CARS) (57) Disclosed is a group of chimeric antigen recep- regulating molecule and optionally reduced by another tors (CARs) consisting of two, three or four CAR mole- regulating molecule, or occurs in the absence of a regu- cules, lating molecule and is reduced by a regulating molecule, wherein the members of the group of CARs can be dif- and ferent or identical in their amino acid sequences to one wherein the ectodomain of each CAR molecule of the another, and group in its prevalent conformation is free of cysteine wherein each of the CAR molecules of the group com- amino acid moieties which are able to form intermolecular prise at least a transmembrane domain and an ectodo- disulphide bonds with other CAR molecules of the -

The Effects of N-3 Fatty Acids and Bexarotene on Breast Cancer Cell Progression
Journal of Cancer Therapy, 2011, 2, 710-714 doi:10.4236/jct.2011.25096 Published Online December 2011 (http://www.SciRP.org/journal/jct) The Effects of n-3 Fatty Acids and Bexarotene on Breast Cancer Cell Progression Jessica Trappmann, Susan N. Hawk Department of Nutrition, Exercise and Health Sciences, Central Washington University, Ellensburg, USA. E-mail: [email protected] Received September 28th, 2011; revised October 25th, 2011; accepted November 7th, 2011. ABSTRACT Breast cancer cell growth can be inhibited in vivo by retinoid X receptor (RXR) specific retinoids. In both animal and cell culture studies, omega-3 fatty acids share growth regulatory effects similar to those of RXR specific retinoids (rex- inoids). One synthetic rexinoid, bexarotene (LCD 1069, Targretin), is used clinically to treat cancer patients. Of con- cern is that some patients are unable to tolerate high doses of such treatment drugs. We hypothesized that n-3 fatty ac- ids and bexarotene may work synergistically to slow breast cancer cell growth. To test our hypothesis, we used MCF-7 human mammary carcinoma cells and an in vitro cell culture model. We investigated the relationship between the omega-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) alone and in conjunction with bex- arotene in slowing MCF-7 cell growth. Following a 72 hr incubation with the respective treatments, bexarotene en- hanced cell growth (p < 0.05) while DHA showed a strong growth inhibitory effect which was not enhanced by the ad- dition of bexarotene (p < 0.05). EPA alone was not effective in altering cell growth (p < 0.05). -

Retinoid-Induced Apoptosis in Normal and Neoplastic Tissues
Cell Death and Differentiation (1998) 5, 11 ± 19 1998 Stockton Press All rights reserved 13509047/98 $12.00 Review Retinoid-induced apoptosis in normal and neoplastic tissues Laszlo Nagy1,3,4, Vilmos A. Thomazy1, Richard A. Heyman2 retinoic acid receptor (RAR), which belongs to the superfamily and Peter J.A. Davies1,3 of ligand-activated transcription factors (nuclear receptors) revolutionized our understanding as to how retinoids exert 1 Department of Pharmacology, University of Texas-Houston, Medical School, their pleiotropic effects (for reviews see Chambon (1996); Houston, Texas 77225 USA Mangelsdorf et al (1994)). Members of the nuclear receptor 2 Ligand Pharmaceuticals, San Diego, California, 92121 USA superfamily mediate the biological effects of many hormones, 3 Corresponding author: PJAD, tel: 713-500-7480; fax: 713-500-7455; vitamins and drugs (i.e. steroid hormones, thyroid hormones, e-mail: [email protected] 4 vitamin D, prostaglandin-J (PG-J ) and drugs that activate Present address for correspondence: The Salk Institute for Biological Studies, 2 2 Gene Expression Laboratory, La Jolla, California 92037; peroxisomal proliferation). There are two families of retinoid tel: (619) 453-4100 fax:(619) 455-1349; e-mail: [email protected] receptors, Retinoid X Receptors (RXRs) that bind 9-cis retinoic acid (9-cis RA) and Retinoic Acid Receptors (RARs) Received 18.8.97; revised 19.9.97; accepted 22.9.97 that bind both 9-cis RA and all-trans retinoic acid (ATRA) (for Edited by M. Piacentini reviews see Chambon 1996; Mangelsdorf et al, 1994)). Each of these receptor families includes at least three distinct genes, (RARa,b and g; RXRa,b and g) that through differential Abstract promoter usage and alternative splicing, give rise to a large number of distinct retinoid receptor proteins (for reviews see Vitamin A and its derivatives (collectively referred to as Chambon 1996; Mangelsdorf et al, 1994). -

Biological Activity and Metabolism of the Retinoid Axerophthene (Vitamin a Hydrocarbon)
[CANCER RESEARCH 38, 1734-1738, June 1978] Biological Activity and Metabolism of the Retinoid Axerophthene (Vitamin A Hydrocarbon) Dianne L. Newton, Charles A. Frolik, Anita B. Roberts, Joseph M. Smith, Michael B. Sporn, Axel NUrrenbach, and Joachim Paust National Cancer Institute, Bethesda, Maryland 20014 [D. L. N., C. A. F., A. B. R., J. M. S., M. B. S.], and BASF Aktiengesellschaft, 6700 Ludwigshafen am Rhein, Germany ¡A.N., J. P] ABSTRACT ity and properties of this molecule. In this study we report a detailed investigation of the biological activity and metabo Biological properties of axerophthene, the hydrocarbon lism of axerophthene. Subsequent studies will deal with the analog of retino!, have been studied both in vitro and in possible effectiveness of this compound for prevention of vivo. In trachea! organ culture axerophthene reversed experimental breast cancer. keratinization caused by deficiency of retinoid in the culture medium; its potency was of the same order of magnitude as that of retinyl acetate. Axerophthene sup MATERIALS AND METHODS ported growth in hamsters fed vitamin A-deficient diets although less effectively than did retinyl acetate. Axer Axerophthene was synthesized as follows: 1100 g (1.75 ophthene was considerably less toxic than was retinyl mol) of crystalline all-E-retinyltriphenylphosphonium bisul acetate when administered repeatedly in high doses to fate (16) were dissolved in 1500 ml of dimethylformamide at rats. Administration of an equivalent p.o. dose of axer about 25°.A solution of 140 g (3.5 mol) of sodium hydroxide ophthene caused much less deposition of retinyl palmi- in 1100 ml of water was added while the temperature of the tate in the liver than did the same dose of retinyl acetate, mixture was kept at about 25°.After being stirred for 3 hr at while a greater level of total retinoid was found in the about 25°,the mixture was extracted with n-hexane (3 x mammary gland after administration of axerophthene. -

Disturbed Vitamin a Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD)
nutrients Review Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD) Ali Saeed 1,2, Robin P. F. Dullaart 3, Tim C. M. A. Schreuder 1, Hans Blokzijl 1 and Klaas Nico Faber 1,4,* 1 Department of Gastroenterology and Hepatology, University Medical Center Groningen, University of Groningen, 9713 GZ Groningen, The Netherlands; [email protected] (A.S.); [email protected] (T.C.M.A.S.); [email protected] (H.B.) 2 Institute of Molecular Biology & Bio-Technology, Bahauddin Zakariya University, Multan 60800, Pakistan 3 Department of Endocrinology, University Medical Center Groningen, University of Groningen, 9713 GZ Groningen, The Netherlands; [email protected] 4 Department of Laboratory Medicine, University Medical Center Groningen, University of Groningen, 9713 GZ Groningen, The Netherlands * Correspondence: [email protected]; Tel.: +31-(0)5-0361-2364; Fax: +31-(0)5-0361-9306 Received: 7 November 2017; Accepted: 19 December 2017; Published: 29 December 2017 Abstract: Vitamin A is required for important physiological processes, including embryogenesis, vision, cell proliferation and differentiation, immune regulation, and glucose and lipid metabolism. Many of vitamin A’s functions are executed through retinoic acids that activate transcriptional networks controlled by retinoic acid receptors (RARs) and retinoid X receptors (RXRs).The liver plays a central role in vitamin A metabolism: (1) it produces bile supporting efficient intestinal absorption of fat-soluble nutrients like vitamin A; (2) it produces retinol binding protein 4 (RBP4) that distributes vitamin A, as retinol, to peripheral tissues; and (3) it harbors the largest body supply of vitamin A, mostly as retinyl esters, in hepatic stellate cells (HSCs).