Research Article P4-Decomposition of Line
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Decomposition of Wheel Graphs Into Stars, Cycles and Paths
Malaya Journal of Matematik, Vol. 9, No. 1, 456-460, 2021 https://doi.org/10.26637/MJM0901/0076 Decomposition of wheel graphs into stars, cycles and paths M. Subbulakshmi1 and I. Valliammal2* Abstract Let G = (V;E) be a finite graph with n vertices. The Wheel graph Wn is a graph with vertex set fv0;v1;v2;:::;vng and edge-set consisting of all edges of the form vivi+1 and v0vi where 1 ≤ i ≤ n, the subscripts being reduced modulo n. Wheel graph of (n + 1) vertices denoted by Wn. Decomposition of Wheel graph denoted by D(Wn). A star with 3 edges is called a claw S3. In this paper, we show that any Wheel graph can be decomposed into following ways. 8 (n − 2d)S ; d = 1;2;3;::: if n ≡ 0 (mod 6) > 3 > >[(n − 2d) − 1]S3 and P3; d = 1;2;3::: if n ≡ 1 (mod 6) > <[(n − 2d) − 1]S3 and P2; d = 1;2;3;::: if n ≡ 2 (mod 6) D(Wn) = . (n − 2d)S and C ; d = 1;2;3;::: if n ≡ 3 (mod 6) > 3 3 > >(n − 2d)S3 and P3; d = 1;2;3::: if n ≡ 4 (mod 6) > :(n − 2d)S3 and P2; d = 1;2;3;::: if n ≡ 5 (mod 6) Keywords Wheel Graph, Decomposition, claw. AMS Subject Classification 05C70. 1Department of Mathematics, G.V.N. College, Kovilpatti, Thoothukudi-628502, Tamil Nadu, India. 2Department of Mathematics, Manonmaniam Sundaranar University, Tirunelveli-627012, Tamil Nadu, India. *Corresponding author: [email protected]; [email protected] Article History: Received 01 November 2020; Accepted 30 January 2021 c 2021 MJM. -

Nilgiris District, Tamil Nadu Connie Smith Tamil Nadu Overview
Nilgiris District, Tamil Nadu Connie Smith Tamil Nadu Overview Tamil Nadu is bordered by Pondicherry, Kerala, Karnataka and Andhra Pradesh. Sri Lanka, which has a significant Tamil minority, lies off the southeast coast. Tamil Nadu, with its traceable history of continuous human habitation since pre-historic times has cultural traditions amongst the oldest in the world. Colonised by the East India Company, Tamil Nadu was eventually incorporated into the Madras Presidency. After the independence of India, the state of Tamil Nadu was created in 1969 based on linguistic boundaries. The politics of Tamil Nadu has been dominated by DMK and AIADMK, which are the products of the Dravidian movement that demanded concessions for the 'Dravidian' population of Tamil Nadu. Lying on a low plain along the southeastern coast of the Indian peninsula, Tamil Nadu is bounded by the Eastern Ghats in the north and Nilgiri, Anai Malai hills and Palakkad (Palghat Gap) on the west. The state has large fertile areas along the Coromandel coast, the Palk strait, and the Gulf of Mannar. The fertile plains of Tamil Nadu are fed by rivers such as Kaveri, Palar and Vaigai and by the northeast monsoon. Traditionally an agricultural state, Tamil Nadu is a leading producer of agricultural products. Tribal Population As per 2001 census, out of the total state population of 62,405,679, the population of Scheduled Castes is 11,857,504 and that of Scheduled Tribes is 651,321. This constitutes 19% and 1.04% of the total population respectively.1 Further, the literacy level of the Adi Dravidar is only 63.19% and that of Tribal is 41.53%. -
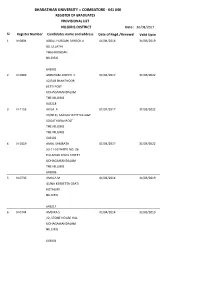
COIMBATORE - 641 046 REGISTER of GRADUATES PROVISIONAL LIST NILGIRIS DISTRICT Date : 30/08/2017 Sl
BHARATHIAR UNIVERSITY :: COIMBATORE - 641 046 REGISTER OF GRADUATES PROVISIONAL LIST NILGIRIS DISTRICT Date : 30/08/2017 Sl. Register Number Candidates name and address Date of Regd./Renewal Valid Upto 1 N-0804 ABDUL HUSSAIN FAROOK A 01/04/2014 31/03/2019 80, ULLATHI THALAKUNDAH NILGIRIS 643005 2 N-0999 ABRAHAM JOSEPH C 01/04/2017 31/03/2022 12/318 SHANTHOOR KETTI POST UDHAGAMANDALAM THE NILGIRIS 643218 3 N-1153 AKILA A 01/04/2017 31/03/2022 20/90 FI, SAKKALHATTY VILLAGE SOGATHORAI POST THE NILGIRIS THE NILGIRIS 643102 4 N-1019 AMAL SAMBATH 01/04/2017 31/03/2022 53-11-53 WARD NO: 26 PILLAIYAR KOVIL STREET UDHAGAMANDALAM THE NILGIRIS 643006 5 N-0736 AMALA M 01/04/2014 31/03/2019 45/NA KERBETTA OSATI KOTAGIRI NILGIRIS 643217 6 N-0744 AMBIKA S 01/04/2014 31/03/2019 22, STONE HOUSE HILL UDHAGAMANDALAM NILGIRIS 643001 BHARATHIAR UNIVERSITY :: COIMBATORE - 641 046 REGISTER OF GRADUATES PROVISIONAL LIST NILGIRIS DISTRICT Date : 30/08/2017 Sl. Register Number Candidates name and address Date of Regd./Renewal Valid Upto 7 N-1240 AMUDESHWARAN A 01/04/2017 31/03/2022 12/40 A1A, ATHIPALLY ROAD KALAMBUZHA GUDALUR THE NILGIRIS 643212 8 N-0750 ANAND S 01/04/2014 31/03/2019 KOOKAL VILL &PO OOTY NILGIRIS 9 N-1008 ANANDARAJ R 01/04/2017 31/03/2022 3/239 B, KADANAD VILLAGE & POST GUDALUR THE NILGIRIS THE NILGIRIS 643206 10 N-1073 ANITHA D 01/04/2017 31/03/2022 18/65 KALLIMARA EPPANADU OOTY THE NILGIRIS 643006 11 N-1246 ANITHA P K 01/04/2017 31/03/2022 1143/6 MARIAMMAN AVENUE, C1 ESTATE, ARUVANKADU, COONOOR, THE NILGIRIS 643202 12 N-1156 ANTIHA A 01/04/2017 31/03/2022 1/191 AARUVA HOSSATTY NADUHATTY KATTABETTU THE NILGIRIS 643214 BHARATHIAR UNIVERSITY :: COIMBATORE - 641 046 REGISTER OF GRADUATES PROVISIONAL LIST NILGIRIS DISTRICT Date : 30/08/2017 Sl. -
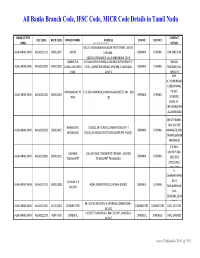
Banks Branch Code, IFSC Code, MICR Code Details in Tamil Nadu
All Banks Branch Code, IFSC Code, MICR Code Details in Tamil Nadu NAME OF THE CONTACT IFSC CODE MICR CODE BRANCH NAME ADDRESS CENTRE DISTRICT BANK www.Padasalai.Net DETAILS NO.19, PADMANABHA NAGAR FIRST STREET, ADYAR, ALLAHABAD BANK ALLA0211103 600010007 ADYAR CHENNAI - CHENNAI CHENNAI 044 24917036 600020,[email protected] AMBATTUR VIJAYALAKSHMIPURAM, 4A MURUGAPPA READY ST. BALRAJ, ALLAHABAD BANK ALLA0211909 600010012 VIJAYALAKSHMIPU EXTN., AMBATTUR VENKATAPURAM, TAMILNADU CHENNAI CHENNAI SHANKAR,044- RAM 600053 28546272 SHRI. N.CHANDRAMO ULEESWARAN, ANNANAGAR,CHE E-4, 3RD MAIN ROAD,ANNANAGAR (WEST),PIN - 600 PH NO : ALLAHABAD BANK ALLA0211042 600010004 CHENNAI CHENNAI NNAI 102 26263882, EMAIL ID : CHEANNA@CHE .ALLAHABADBA NK.CO.IN MR.ATHIRAMIL AKU K (CHIEF BANGALORE 1540/22,39 E-CROSS,22 MAIN ROAD,4TH T ALLAHABAD BANK ALLA0211819 560010005 CHENNAI CHENNAI MANAGER), MR. JAYANAGAR BLOCK,JAYANAGAR DIST-BANGLAORE,PIN- 560041 SWAINE(SENIOR MANAGER) C N RAVI, CHENNAI 144 GA ROAD,TONDIARPET CHENNAI - 600 081 MURTHY,044- ALLAHABAD BANK ALLA0211881 600010011 CHENNAI CHENNAI TONDIARPET TONDIARPET TAMILNADU 28522093 /28513081 / 28411083 S. SWAMINATHAN CHENNAI V P ,DR. K. ALLAHABAD BANK ALLA0211291 600010008 40/41,MOUNT ROAD,CHENNAI-600002 CHENNAI CHENNAI COLONY TAMINARASAN, 044- 28585641,2854 9262 98, MECRICAR ROAD, R.S.PURAM, COIMBATORE - ALLAHABAD BANK ALLA0210384 641010002 COIIMBATORE COIMBATORE COIMBOTORE 0422 2472333 641002 H1/H2 57 MAIN ROAD, RM COLONY , DINDIGUL- ALLAHABAD BANK ALLA0212319 NON MICR DINDIGUL DINDIGUL DINDIGUL -

Mandatory Disclosure
Anna University, Chennai CSI College of Engineering - 7106 1.College Name Of the College CSI COLLEGE OF ENGINEERING Address KETTI POST,THE NILGIRIS Pincode 643215 Year of establishment of the 1998 college Type of the Institution SELF FINANCING Category Of the College CHRISTIAN MINORITY Type of college ENGINEERING Is the College Autonomous NO Is the college Functioning at the YES above said-approved site? Mobile Numbers 9487791110 Telephone Numbers 0423 - 2517581 Other Telephone Numbers 0423 - 2517584,25177474,25178099 Fax Numbers 0423 - 2571699 E mail ID [email protected] Website Address www.csice.edu.in Inclusion under Sections 2(f) and 12(B) of the UGC Act, 1956 Section 2(f) Included Letter No. and date, if included F.No.8-40/2016 & 27-07-2017 Section 12(B) Included Letter No. and date, if included F.NO.8-40/2016 & 27-07-2017 Any other Accreditation / Recognition Date Of Generation 02-01-2020 07:21:48 Page 1 / 1 Anna University, Chennai CSI College of Engineering - 7106 2. Trust and Society Details The Head of the Trust Chairman CHURCH OF SOUTH INDIA TRUST Name Of the Trust/Society ASSOCIATION COIMBATORE DIOCESAN COUNCIL Address of the Registered Office 256 RACE COURSE ROAD COIMBATORE 641018 District Coimbatore Name of the Chairman Mr. TIMOTHY RAVINDER Rt.Rev. Father Name Mr. TIMOTHY Mr. E-mail [email protected] Mobile Number of the Chairman 9487791110 Telephone number 0422 - 2223605 Telephone number - Office 0422 - 2223605 Telephone number - Residence 0423 - 2571699 Registration Number Date Registration Number & Date 112 of 1947-48 31-08-1948 Date Of Generation 02-01-2020 07:22:29 Page 1 / 1 Anna University, Chennai CSI College of Engineering - 7106 5.Planning and Monitoring Board Member Details Present Telephone Mobile Name Position Category Qualification Designation / E-mail ID Address Numbers Numbers Occupation Dr. -

A New Approach to Number of Spanning Trees of Wheel Graph Wn in Terms of Number of Spanning Trees of Fan Graph Fn
[ VOLUME 5 I ISSUE 4 I OCT.– DEC. 2018] E ISSN 2348 –1269, PRINT ISSN 2349-5138 A New Approach to Number of Spanning Trees of Wheel Graph Wn in Terms of Number of Spanning Trees of Fan Graph Fn Nihar Ranjan Panda* & Purna Chandra Biswal** *Research Scholar, P. G. Dept. of Mathematics, Ravenshaw University, Cuttack, Odisha. **Department of Mathematics, Parala Maharaja Engineering College, Berhampur, Odisha-761003, India. Received: July 19, 2018 Accepted: August 29, 2018 ABSTRACT An exclusive expression τ(Wn) = 3τ(Fn)−2τ(Fn−1)−2 for determining the number of spanning trees of wheel graph Wn is derived using a new approach by defining an onto mapping from the set of all spanning trees of Wn+1 to the set of all spanning trees of Wn. Keywords: Spanning Tree, Wheel, Fan 1. Introduction A graph consists of a non-empty vertex set V (G) of n vertices together with a prescribed edge set E(G) of r unordered pairs of distinct vertices of V(G). A graph G is called labeled graph if all the vertices have certain label, and a tree is a connected acyclic graph according to Biggs [1]. All the graphs in this paper are finite, undirected, and simple connected. For a graph G = (V(G), E(G)), a spanning tree in G is a tree which has the same vertex set as G has. The number of spanning trees in a graph G, denoted by τ(G), is an important invariant of the graph. It is also an important measure of reliability of a network. -

On Graph Crossing Number and Edge Planarization∗
On Graph Crossing Number and Edge Planarization∗ Julia Chuzhoyy Yury Makarychevz Anastasios Sidiropoulosx Abstract Given an n-vertex graph G, a drawing of G in the plane is a mapping of its vertices into points of the plane, and its edges into continuous curves, connecting the images of their endpoints. A crossing in such a drawing is a point where two such curves intersect. In the Minimum Crossing Number problem, the goal is to find a drawing of G with minimum number of crossings. The value of the optimal solution, denoted by OPT, is called the graph's crossing number. This is a very basic problem in topological graph theory, that has received a significant amount of attention, but is still poorly understood algorithmically. The best currently known efficient algorithm produces drawings with O(log2 n) · (n + OPT) crossings on bounded-degree graphs, while only a constant factor hardness of approximation is known. A closely related problem is Minimum Planarization, in which the goal is to remove a minimum-cardinality subset of edges from G, such that the remaining graph is planar. Our main technical result establishes the following connection between the two problems: if we are given a solution of cost k to the Minimum Planarization problem on graph G, then we can efficiently find a drawing of G with at most poly(d) · k · (k + OPT) crossings, where d is the maximum degree in G. This result implies an O(n · poly(d) · log3=2 n)-approximation for Minimum Crossing Number, as well as improved algorithms for special cases of the problem, such as, for example, k-apex and bounded-genus graphs. -

Coonoor Club AGM Agenda Dtd 09.09.2019, Accounts for the Year 2018
OOR C N L O U O B C COONOOR CLUB (Registered under the Societies Registration Act of 1975) (S.No 22 of 1991) Members of the Managing Committee Notice of the Annual General Meeting Report of the Managing Committee Audited Accounts List of Members List of Affiliated Clubs ANNUAL ACCOUNTS & REPORT 2018-2019 MEMBERS OF THE MANAGING COMMITTEE PRESIDENT Mr. Ullas Menon COMMITTEE MEMBERS Mr. S.Kumar ( From 24.09.2019 to 09.01.2019 ) Mr. Philip Mathew Mr. G.Nagaraj Mr. N.K.Devaraj Mr. H.S.Mehta Mr. K.Vinod Shenai Mrs. Deepa Jacob ( From 31.01.2019 onwards ) CO-OPTED MEMBERS Mr. S.Rajubettan Mr.Thomas C Oommen CHIEF ADMINISTRATIVE OFFICER Mr. G. Sudheendran BANKERS Central Bank of India, Coonoor IndusInd Bank, Coonoor State Bank of India, Coonoor The South Indian Bank Ltd., Coonoor The Karnataka Bank Ltd., Coonoor IDBI Bank, Coonoor STATUTORY AUDITORS Messrs. L. Ashok & Associates, Coonoor. COONOOR CLUB, COONOOR NOTICE – ANNUAL GENERAL MEETING NOTICE is hereby given that the Annual General Meeting of the Coonoor Club will be held at the Coonoor Club, Club Road, Coonoor 643 101, Nilgiris, Tamil Nadu, on Monday, 30th September, 2019 at 6.30 p.m. By order of the Managing Committee Sd/- Coonoor G. Sudheendran 9th September, 2019 C.A.O. AGENDA 1. To read the notice calling the meeting 2. President’s address 3. To approve and adopt the Minutes of the Annual General Meeting held on 24th September 2018. 4. To approve and adopt the Minutes of the Extraordinary General Meeting held on 24th September 2018. -

Pykara Pykara Is the Name of a Village and River Located 19 Km from Ooty
Pykara Pykara is the name of a village and river located 19 km from Ooty in the Indian State of Tamil Nadu. A boat house on the Pykara reservoir is an added attraction for tourists. Pykara boats of well protected fenced shoals. Pykara waterfalls flows through Murkurti, Pykara and Glen Morgan dams.. Mudumalai Before going to mudumali make sure you read this below point clearly OKDDSDD Obey Speed Limit Keep Noise level down Do not feed wildlife Do not dispose rubbish Shouting, teasing or chasing animals is prohibited Do not get out of your vechile Don't park vehicle on the road sideThose who violate the regulations will be prosecuted and punished according to law Dodebetta Doddabetta is the highest mountain in the Nilgiri Hills, at 2637 metres (8650 feet). There is a reserved forest area around the peak. It is 9 km from Ooty,on the Ooty-Kotagiri Road in the Nilgiris District of Tamil Nadu, South India. It is a popular tourist attraction with road access to the summit. The Chamundi Hills can be viewed from the peak.There is an observatory at the top of Doddabetta with two telescopes available for the public to enjoy the magnificent panoramic view of the whole district. The beautiful valley, plains of Coimbatore and the flat highlands of Mysore are visible from this point. Tea estate view point The Nilgiri region is remowned for its tea and most of the areas in ooty is sorrounded with the tea plantation. The tea estate or plantation is one of the tourist attraction and the tourist enjoy to go for a walk in the tea estate which gives them peaceful. -

GIS DATA BASE GENERATION on LANDSLIDES by TRACING the HISTORICAL LANDSLIDE LOCATIONS in NILGIRI DISTRICT, SOUTH INDIA Gurugnanam
International Journal of Remote Sensing & Geoscience (IJRSG) www.ijrsg.com GIS DATA BASE GENERATION ON LANDSLIDES BY TRACING THE HISTORICAL LANDSLIDE LOCATIONS IN NILGIRI DISTRICT, SOUTH INDIA Gurugnanam. B., Centre for Applied Geology, Gandhigram Rural Institute-Deemed University ; Tamilnadu Arun Kumar. M. Centre for Applied Geology, Gandhigram Rural Institute – Deemed University; Tamilnadu Bairavi, S. Spatial Technology, Department of Rural Development, Gandhigram Rural Institute – Deemed University: Tamilnadu Vasudevan. S, Centre for Geographic Information Technologies, Department of Geology, Bharathidasan University, Tamilnadu [email protected] Abstract Methodology Generating GIS database for landslide locations is attempted in The landslide locations were collected for the miscellaneous the present paper. Literature surveys were conducted to take the report from the Geological Survey of India. The location data information on landslides. The existing landslide locations were were also collected from the literature survey 50 numbers of pa- collected from the Geological Survey of India Miscellaneous pers were collected from the Nilgiri regions. The details were publications and other literature survey. This information was taken from the existing reference. The data were formulated to collected in the form of latitude and longitude data source. This give the latitude and longitude information point information in a data were given as input in GIS. To understand the event in year GIS. These were tabulated with the information like landslide wise information is spatially given to understand the landslide locations, latitude, longitude, Year of occurrence (Table.1). distribution in the study area. Major landslides were recorded in Using the latitude and longitude information, the details were the year 2009 with a human death loss of around 50 numbers in given as an input to GIS platform. -

An Introduction to Algebraic Graph Theory
An Introduction to Algebraic Graph Theory Cesar O. Aguilar Department of Mathematics State University of New York at Geneseo Last Update: March 25, 2021 Contents 1 Graphs 1 1.1 What is a graph? ......................... 1 1.1.1 Exercises .......................... 3 1.2 The rudiments of graph theory .................. 4 1.2.1 Exercises .......................... 10 1.3 Permutations ........................... 13 1.3.1 Exercises .......................... 19 1.4 Graph isomorphisms ....................... 21 1.4.1 Exercises .......................... 30 1.5 Special graphs and graph operations .............. 32 1.5.1 Exercises .......................... 37 1.6 Trees ................................ 41 1.6.1 Exercises .......................... 45 2 The Adjacency Matrix 47 2.1 The Adjacency Matrix ...................... 48 2.1.1 Exercises .......................... 53 2.2 The coefficients and roots of a polynomial ........... 55 2.2.1 Exercises .......................... 62 2.3 The characteristic polynomial and spectrum of a graph .... 63 2.3.1 Exercises .......................... 70 2.4 Cospectral graphs ......................... 73 2.4.1 Exercises .......................... 84 3 2.5 Bipartite Graphs ......................... 84 3 Graph Colorings 89 3.1 The basics ............................. 89 3.2 Bounds on the chromatic number ................ 91 3.3 The Chromatic Polynomial .................... 98 3.3.1 Exercises ..........................108 4 Laplacian Matrices 111 4.1 The Laplacian and Signless Laplacian Matrices .........111 4.1.1 -

COLORING GRAPHS USING TOPOLOGY 11 Realized by Tutte Who Called an Example of a Disc G for Which the Boundary Has Chromatic Number 4 a Chromatic Obstacle
COLORING GRAPHS USING TOPOLOGY OLIVER KNILL Abstract. Higher dimensional graphs can be used to color 2- dimensional geometric graphs G. If G the boundary of a three dimensional graph H for example, we can refine the interior until it is colorable with 4 colors. The later goal is achieved if all inte- rior edge degrees are even. Using a refinement process which cuts the interior along surfaces S we can adapt the degrees along the boundary S. More efficient is a self-cobordism of G with itself with a host graph discretizing the product of G with an interval. It fol- lows from the fact that Euler curvature is zero everywhere for three dimensional geometric graphs, that the odd degree edge set O is a cycle and so a boundary if H is simply connected. A reduction to minimal coloring would imply the four color theorem. The method is expected to give a reason \why 4 colors suffice” and suggests that every two dimensional geometric graph of arbitrary degree and ori- entation can be colored by 5 colors: since the projective plane can not be a boundary of a 3-dimensional graph and because for higher genus surfaces, the interior H is not simply connected, we need in general to embed a surface into a 4-dimensional simply connected graph in order to color it. This explains the appearance of the chromatic number 5 for higher degree or non-orientable situations, a number we believe to be the upper limit. For every surface type, we construct examples with chromatic number 3; 4 or 5, where the construction of surfaces with chromatic number 5 is based on a method of Fisk.