July 26, 2021 CMS # 608717 Andy Jassy, CEO Amazon.Com, Inc. 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
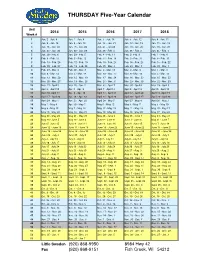
Thursday Calendar 20142018
THURSDAY Five-Year Calendar Unit 2014 20152016 2017 2018 Week # 1 Jan. 2 - Jan. 9 Jan. 1 - Jan. 8 Jan. 7 - Jan. 14 Jan. 5 - Jan. 12 Jan. 4 - Jan. 11 2 Jan. 9 - Jan. 16 Jan. 8 - Jan. 15 Jan. 14 - Jan. 21 Jan. 12 - Jan. 19 Jan. 11 - Jan. 18 3 Jan. 16 - Jan. 23 Jan. 15 - Jan. 22 Jan. 21 - Jan 28 Jan. 19 - Jan. 26 Jan. 18 - Jan. 25 4 Jan. 23 - Jan. 30 Jan. 22 - Jan. 29 Jan. 28 - Feb. 4 Jan. 26 - Feb. 2 Jan. 25 - Feb. 1 5 Jan. 30 - Feb. 6 Jan. 29 - Feb. 5 Feb. 4 - Feb. 11 Feb. 2- Feb. 9 Feb. 1 - Feb. 8 6 Feb. 6 - Feb. 13 Feb. 5 - Feb. 12 Feb. 11 - Feb. 18 Feb. 9 - Feb. 16 Feb. 8 - Feb. 15 7 Feb. 13 - Feb. 20 Feb. 12 - Feb. 19 Feb. 18 - Feb. 25 Feb. 16 - Feb. 23 Feb. 15 - Feb. 22 8 Feb. 20 - Feb. 27 Feb. 19 - Feb. 26 Feb. 25 - Mar. 3 Feb. 23 - Mar. 2 Feb. 22 - Mar. 1 9 Feb. 27 - Mar. 6 Feb. 26 - Mar. 5 Mar. 3 - Mar. 10 Mar. 2 - Mar. 9 Mar. 1 - Mar. 8 10 Mar. 6 - Mar. 13 Mar. 5 - Mar. 12 Mar. 10 - Mar. 17 Mar. 9 - Mar. 16 Mar. 5 - Mar. 15 11 Mar. 13 - Mar. 20 Mar. 12 - Mar. 19 Mar. 17 - Mar. 24 Mar. 16 - Mar. 23 Mar. 15 - Mar. 22 12 Mar. 20 - Mar. 27 Mar. 19 - Mar. 26 Mar. 24 - Mar. 31 Mar. 23 - Mar. 30 Mar. 22 - Mar. -

2021 Pay Dates
2021 PAY DATES**** 2021 STATE 2021 FEDERAL IRS $100,000 Next-Day WAC 82-50-021 HOLIDAYS***** HOLIDAYS Deposit Rule date* WAC 357-31-005 Federal Holiday Schedules IRS Publication 15 Monday, January 11, 2021 Friday – January 1, 2021 Friday – January 1, 2021 Tuesday – January 12, 2021 Monday – January 25, 2021 Monday - January 18, 2021 Monday - January 18, 2021 Tuesday – January 26, 2021 Wednesday – February 10, 2021 Thursday – February 11, 2021 Thursday - February 25, 2021 Monday - February 15, 2021 Monday - February 15, 2021 Friday – February 26, 2021 Wednesday - March 10, 2021 Thursday – March 11, 2021 Thursday - March 25, 2021 Friday – March 26, 2021 Friday - April 9, 2021 Monday – April 12, 2021 Monday - April 26, 2021 Tuesday – April 27, 2021 Monday - May 10, 2021 Tuesday - May 11, 2021 Tuesday - May 25, 2021 Monday - May 31, 2021 Monday - May 31, 2021 Wednesday - May 26, 2021 Thursday - June 10, 2021 Friday - June 11, 2021 Friday - June 25, 2021 Monday - June 28, 2021 Friday - July 9, 2021 Monday - July 5, 2021 Monday - July 5, 2021 Monday - July 12, 2021 Monday - July 26, 2021 Tuesday - July 27, 2021 Tuesday – August 10, 2021 Wednesday – August 11, 2021 Wednesday – August 25, 2021 Thursday - August 26, 2021 Friday – September 10, 2021 Monday - September 6, 2021 Monday - September 6, 2021 Monday – September 13, 2021 Friday – September 24, 2021 Monday – September 27, 2021 Friday – October 8, 2021****** Monday – October 11, 2021 Tuesday – October 12, 2021** Monday – October 25, 2021 Tuesday - October 26, 2021 Wednesday – November 10, 2021 Thursday - November 11, 2021 Thursday - November 11, 2021 Friday - November 12, 2021** Wednesday - November 24, 2021 Thursday & Friday – November 25 & 26, Thursday - November 25, 2021 Friday – November 26, 2021** 2021 Friday - December 10, 2021 Monday – December 13, 2021 Thursday - December 23, 2021*** Friday – December 24, 2021 Saturday – December 25, 2021 Friday – December 24, 2021** Friday – December 31,2021 (Holiday for January 1, 2022) *For agencies with a payday deposit liability greater than $100,000. -

Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No
Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No. 33 Class & Market Administrator Payment Dates for Producer Milk Component Final Pool Producer Advance Prices Payment Dates Final Payment Due Partial Payment Due Pool Month Prices Release Date Payrolls Due & Pricing Factors PSF, Admin., MS Cooperative Nonmember Cooperative Nonmember January February 3 * February 13 February 22 December 23, 2020 February 16 ** February 16 February 17 Janaury 25 January 26 February March 3 * March 13 March 22 January 21 * March 15 March 16 March 17 February 25 February 26 March March 31 * April 13 April 22 February 18 * April 15 April 16 April 19 ** March 25 March 26 April May 5 May 13 May 22 March 17 * May 17 ** May 17 ** May 17 April 26 ** April 26 May June 3 * June 13 June 22 April 21 * June 15 June 16 June 17 May 25 May 26 June June 30 * July 13 July 22 May 19 * July 15 July 16 July 19 ** June 25 June 28 ** July August 4 * August 13 August 22 June 23 August 16 ** August 16 August 17 July 26 ** July 26 August September 1 * September 13 September 22 July 21 * September 15 September 16 September 17 August 25 August 26 September September 29 * October 13 October 22 August 18 * October 15 October 18 ** October 18 ** September 27 ** September 27 ** October November 3 * November 13 November 22 September 22 * November 15 November 16 November 17 October 25 October 26 November December 1 * December 13 December 22 October 20 * December 15 December 16 December 17 November 26 ** November 26 December January 5, 2022 January 13, 2022 January 22, 2022 November 17 * January 18, 2022 ** January 18, 2022 ** January 18, 2022 ** December 27 ** December 27 ** * If the release date does not fall on the 5th (Class & Component Prices) or 23rd (Advance Prices & Pricing Factors), the most current release preceding will be used in the price calculation. -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -
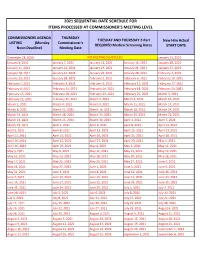
2021 Sequential Date List
2021 SEQUENTIAL DATE SCHEDULE FOR ITEMS PROCESSED AT COMMISSIONER'S MEETING LEVEL COMMISSIONERS AGENDA THURSDAY TUESDAY AND THURSDAY 2-Part New Hire Actual LISTING (Monday Commissioner's REQUIRED Medical Screening Dates START DATE Noon Deadline) Meeting Date December 28, 2020 NO MEETING SCHEDULED January 13, 2021 January 4, 2021 January 7, 2021 January 12, 2021 January 14, 2021 January 20, 2021 January 11, 2021 January 14, 2021 January 19, 2021 January 21, 2021 January 27, 2021 January 18, 2021 January 21, 2021 January 26, 2021 January 28, 2021 February 3, 2021 January 25, 2021 January 28, 2021 February 2, 2021 February 4, 2021 February 10, 2021 February 1, 2021 February 4, 2021 February 9, 2021 February 11, 2021 February 17, 2021 February 8, 2021 February 11, 2021 February 16, 2021 February 18, 2021 February 24, 2021 February 15, 2021 February 18, 2021 February 23, 2021 February 25, 2021 March 3, 2021 February 22, 2021 February 25, 2021 March 2, 2021 March 4, 2021 March 10, 2021 March 1, 2021 March 4, 2021 March 9, 2021 March 11, 2021 March 17, 2021 March 8, 2021 March 11, 2021 March 16, 2021 March 18, 2021 March 24, 2021 March 15, 2021 March 18, 2021 March 23, 2021 March 25, 2021 March 31, 2021 March 22, 2021 March 25, 2021 March 30, 2021 April 1, 2021 April 7, 2021 March 29, 2021 April 1, 2021 April 6, 2021 April 8, 2021 April 14, 2021 April 5, 2021 April 8, 2021 April 13, 2021 April 15, 2021 April 21, 2021 April 12, 2021 April 15, 2021 April 20, 2021 April 22, 2021 April 28, 2021 April 19, 2021 April 22, 2021 April 27, 2021 April -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018 -

2021 Working Day Calendar-5 Day-Alternative Format
2021 Working Days Calendar – 5 day The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 Non-working Day Saturday, January 2, 2021 Non-working Day Sunday, January 3, 2021 Non-working Day Monday, January 4, 2021 938 Tuesday, January 5, 2021 939 Wednesday, January 6, 2021 940 Thursday, January 7, 2021 941 Friday, January 8, 2021 942 Saturday, January 9, 2021 Non-working Day Sunday, January 10, 2021 Non-working Day Monday, January 11, 2021 943 Tuesday, January 12, 2021 944 Wednesday, January 13, 2021 945 Thursday, January 14, 2021 946 Friday, January 15, 2021 947 Saturday, January 16, 2021 Non-working Day Sunday, January 17, 2021 Non-working Day Monday, January 18, 2021 Non-working Day Tuesday, January 19, 2021 948 Wednesday, January 20, 2021 949 Thursday, January 21, 2021 950 Friday, January 22, 2021 951 Saturday, January 23, 2021 Non-working Day Sunday, January 24, 2021 Non-working Day Monday, January 25, 2021 952 Tuesday, January 26, 2021 953 Wednesday, January 27, 2021 954 Thursday, January 28, 2021 955 Friday, January 29, 2021 956 Saturday, January 30, 2021 Non-working Day Sunday, January 31, 2021 Non-working Day Monday, February 1, 2021 957 Tuesday, February 2, 2021 958 Wednesday, February 3, -
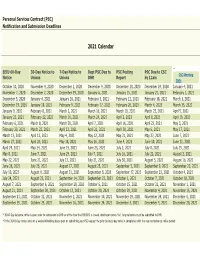
2021 Calendar
Personal Services Contract (PSC) Notification and Submission Deadlines 2021 Calendar SEIU 60-Day 30-Day Notice to 7-Day Notice to Dept PSC Due to PSC Posting PSC Due to CSC CSC Meeting Notice Unions Unions DHR Report by 11am Date October 10, 2020 November 9, 2020 December 1, 2020 December 9, 2020 December 18, 2020 December 24, 2020 January 4, 2021 November 7, 2020 December 7, 2020 December 29, 2020 January 6, 2021 January 15, 2021 January 21, 2021 February 1, 2021 December 5, 2020 January 4, 2021 January 26, 2021 February 3, 2021 February 12, 2021 February 18, 2021 March 1, 2021 December 19, 2020 January 18, 2021 February 9, 2021 February 17, 2021 February 26, 2021 March 4, 2021 March 15, 2021 January 9, 2021 February 8, 2021 March 2, 2021 March 10, 2021 March 19, 2021 March 25, 2021 April 5, 2021 January 23, 2021 February 22, 2021 March 16, 2021 March 24, 2021 April 2, 2021 April 8, 2021 April 19, 2021 February 6, 2021 March 8, 2021 March 30, 2021 April 7, 2021 April 16, 2021 April 22, 2021 May 3, 2021 February 20, 2021 March 22, 2021 April 13, 2021 April 21, 2021 April 30, 2021 May 6, 2021 May 17, 2021 March 13, 2021 April 12, 2021 May 4, 2021 May 12, 2021 May 21, 2021 May 27, 2021 June 7, 2021 March 27, 2021 April 26, 2021 May 18, 2021 May 26, 2021 June 4, 2021 June 10, 2021 June 21, 2021 April 24, 2021 May 24, 2021 June 15, 2021 June 23, 2021 July 2, 2021 July 8, 2021 July 19, 2021 May 8, 2021 June 7, 2021 June 29, 2021 July 7, 2021 July 16, 2021 July 22, 2021 August 2, 2021 May 22, 2021 June 21, 2021 July 13, 2021 July 21, 2021 July -

Utility Billing Dates 2021
2021 Reading & Billing Schedule for Website.xlsx Billing Month Cycle Start Read date Finish read date Billing Date Due Date & Bank Draft Date Penalty Date Pay by dates to avoid cut off Cut off date January 1 Monday, December 21, 2020 Thursday, December 24, 2020 Thursday, January 7, 2021 Thursday, January 28, 2021 Tuesday, February 2, 2021 Tuesday, February 16, 2021 Wednesday, February 17, 2021 2 Monday, December 28, 2020 Thursday, December 31, 2020 Thursday, January 14, 2021 Thursday, February 4, 2021 Tuesday, February 9, 2021 Tuesday, February 23, 2021 Wednesday, February 24, 2021 3 Monday, January 4, 2021 Thursday, January 7, 2021 Thursday, January 21, 2021 Thursday, February 11, 2021 Tuesday, February 16, 2021 Tuesday, March 2, 2021 Wednesday, March 3, 2021 4 Monday, January 11, 2021 Thursday, January 14, 2021 Thursday, January 28, 2021 Thursday, February 18, 2021 Tuesday, February 23, 2021 Tuesday, March 9, 2021 Wednesday, March 10, 2021 February 1 Tuesday, January 19, 2021 Friday, January 22, 2021 Thursday, February 4, 2021 Thursday, February 25, 2021 Tuesday, March 2, 2021 Tuesday, March 16, 2021 Wednesday, March 17, 2021 2 Monday, January 25, 2021 Thursday, January 28, 2021 Thursday, February 11, 2021 Thursday, March 4, 2021 Tuesday, March 9, 2021 Tuesday, March 23, 2021 Wednesday, March 24, 2021 3 Monday, February 1, 2021 Thursday, February 4, 2021 Thursday, February 18, 2021 Thursday, March 11, 2021 Tuesday, March 16, 2021 Tuesday, March 30, 2021 Wednesday, March 31, 2021 4 Monday, February 8, 2021 Thursday, February 11, 2021 -

Citrus College COVID-19 Exposure Notification, July 26 2021
CORONAVIRUS (COVID-19) EXPOSURE NOTIFICATION Email to students and employees – July 26, 2021 DATE: July 26, 2021 FROM: Robert Sammis and the COVID-19 Workgroup RE: COVID-19 Exposure Notification Dear Citrus College Students and Colleagues, I am writing to inform you that the college has recently received information about a confirmed case of Coronavirus 2019 (COVID-19) in at least one student located in the football practice area. Those who may have been in the area or had contact with the individual have been notified, and cleaning and disinfecting of the exposed location are in progress. If you were in this identified area on July 19, 20, 21, or 22, and you have not already been contacted by the college, please let me know via email, [email protected], and I will provide you with further instructions. The following guidelines are offered as precautionary measures: Wear a face covering (mask) as currently required by the County of Los Angeles Department of Public Health while indoors regardless of vaccination status. Stay home when you are sick. Anyone with symptoms consistent with COVID-19 should remain at home in isolation for a minimum of 10 days plus at least three days after the resolution of fever (without fever- reducing medication) and improvement in other symptoms. District provided COVID-19 sick leave (80 hours) is available to full-time employees in addition to regular sick leave. Wash your hands often with soap and water for at least 20 seconds. If soap and water are not available, use alcohol-based hand sanitizers that contain at least 60% alcohol.