Neuroimaging of Phakomatoses Doris D.M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aborted Sudden Death in a Young Male SELF ASSESSMENT
664 Postgrad Med J 2003;79:664–666 Postgrad Med J: first published as 10.1136/pmj.79.937.666 on 3 December 2003. Downloaded from SELF ASSESSMENT ANSWERS Aborted sudden death in a Q2: What is the pathophysiological basisofthiscondition? What further young male diagnostic tests would you consider doing in this patient? Q1: What is the ECG (fig 1; p 660) Genetic studies have shown that Brugada diagnosis? Why is it important to syndrome and chromosome 3-linked long QT recognise this condition? syndrome (LQT3) are allelic disorders of the The ECG done on his arrival at the emergency cardiac channel gene (SCN5A, 3p21). The room (see questions) shows (i) sinus tachy- inheritance is autosomal dominant with cardia, (ii) a QRS complex that ends with a variable penetrance. The SCN5A gene codes positive deflection (or prominent J wave) for the alpha subunit of the sodium channel. that is, a rsR9 pattern in V1 and V2, and (iii) Mutations of this gene results in abnormal- an elevated downsloping ST segment ending ities of the sodium channel, with abnormal in a small negative T-wave deflection. This ion conductance patterns and can be demon- ECG pattern in someone with a history of strated in up to 25% Brugada syndrome syncopy and documented ventricular fibrilla- cases.235 Brugada-type downsloping ST seg- tion/aborted sudden death, is most consistent ment is a normal feature of the ECG in some with the eponymous Brugada syndrome. rodents, whereas in higher mammals, the ST Described first in 1992 by Brugada and segment is usually isoelectric in the normal Brugada, Brugada syndrome is an inherited state. -

TSC Facts Countdown for 15 Days in May: 1. Tuberous Sclerosis Complex
TSC Facts Countdown for 15 days in May: 1. Tuberous sclerosis complex (TSC) is a genetic disorder with no cure that causes non-cancerous tumors to form in vital organs. 2. Tuberous sclerosis complex (TSC) is estimated to affect 1 in 6,000 live births. Globally, one million individuals have TSC, making it as common as cystic fibrosis or amyotrophic lateral sclerosis (ALS). 3. Approximately ⅔ of individuals diagnosed with tuberous sclerosis complex (TSC) have no family history. The remaining ⅓ of individuals diagnosed with TSC have a parent who also has TSC. 4. If one parent is diagnosed with tuberous sclerosis complex (TSC), the probability of his or her children inheriting the disease is 50%. If parents are unaffected by TSC and have one child with TSC, the probability of having another child with TSC is around 1-2%. 5. Tuberous sclerosis complex (TSC) is the leading genetic cause of both epilepsy and autism spectrum disorders. Seizures occur in approximately 85% of individuals with TSC and intellectual disabilities are found in 45-60%. 6. Approximately 98% of individuals experience one or more skin manifestations (such as angiofibromas) of tuberous sclerosis complex (TSC). 7. Up to 60% of individuals experience kidney involvement with tuberous sclerosis complex (TSC). 8. Tuberous sclerosis complex (TSC) affects men and women in equal numbers and occurs in all races and ethnic groups. 9. Tuberous sclerosis complex (TSC) affects everyone differently; some may have mild symptoms while others are severely impacted. TSC symptoms often vary over a person’s lifetime—someone who has few childhood symptoms may still have severe health problems later in life. -

Tuberous Sclerosis in Early Infancy: a Case Report H
Brief Report Tuberous Sclerosis in Early Infancy: A Case Report H. John Blossom, MD Fresno, California The diagnosis of tuberous sclerosis in an infant was de sclerosis contributed to the delay. A brief review of the layed by 3 months. Failure to take an adequate padent case and the diagnosis of tuberous sclerosis is presented. history because of a language barrier between parents and Key words: Tuberous sclerosis; seizures; infant care. caregivers and to observe the classic stigmata of tuberous / Pam Proa 1993; 36:344-346. The occurrence of seizures in an infant is a frightening evaluation was apparently delayed because of a diagnosis experience for parents and physicians alike. In this case of persistent otitis media, which required repeat admin report important historical information and unique phys istrations of antibiotics. ical findings arc described that helped determine the During the infant’s 4th month of life, several visits cause of seizures in a 4-month-old infant. to physicians were made for fussiness and fever. Otitis media was diagnosed several times. In addition, the par ents observed and reported an abnormality in the baby’s Case Report behavior that they described in Spanish as ataques, a term commonly used to describe fits or seizures. During clin The infant weighed 9 lb 3 oz at birth. His mother had an ical encounters interpreters translated conversations be uncomplicated prenatal course and an uneventful deliv tween the physicians and the parents. Because the ataques ery. The infant’s Apgar scores were 8/8 at 1 and 5 were of very brief duration and no abnormal physical minutes. -

Front Office Staff Flash Card
Tuberous Sclerosis Complex: An Overview Tuberous Sclerosis Complex (TSC) is a Approximately Multiorgan Genetic Disorder 50,000 people in the United States • It is characterized by the have TSC1 formation of hamartomas, which are noncancerous tumor-like masses2,5 TSC occurs in all races • These tumors can form and ethnic groups, and in in major organs including both genders2 the brain, skin, eyes, and kidneys. Tumors in the heart often occur in children, while lung tumors can The disease occur in adults2,5,6 affects an estimated • Symptoms of TSC can range 1 in 6,000 from mild to severe and can 2 newborns change over time2,6 TSC may not be noticeable. - Because symptoms vary and Diseases with similar may not be immediately recognized US prevalence rates by a health care provider, TSC is include cystic fibrosis often undiagnosed for years (approximately 30,000 people) and amyotrophic Depending on the body organs affected by TSC, lateral sclerosis (ALS), different specialists may be involved, such as a: or Lou Gehrig’s disease (up to approximately Nephrologist 3,4 or for kidney manifestations, such as renal 30,000 people) urologist angiomyolipoma Neurologist for brain manifestations, such as subependymal nodules (SENs) and About 1/3 subependymal giant-cell astrocytomas (SEGAs) of all people with TSC genetically inherit the Dermatologist for skin manifestations disease, while in the remaining individuals, Pulmonologist for lung complications, such as the disease is acquired lymphangioleiomyomatosis (LAM) as a result of spontaneous genetic -

** No Patient Handout Darier Disease In
** no patient handout Darier disease in Synopsis Darier disease, also known as keratosis follicularis or Darier-White disease, is an autosomal dominantly inherited disease caused by mutations in the ATP2A2 gene, which encodes a sarco / endoplasmic reticulum calcium-ATPase pump (SERCA2). Although disease penetrance is high, expression is variable, and sporadic mutations may occur. There is no sex predilection. Darier disease presents in early adolescence to mid-adult life, with peak onset in the second decade of life. The disease manifests with greasy, hyperkeratotic papules in a seborrheic distribution, along with palmoplantar pits, acrokeratosis verruciformis-like papules, and characteristic nail findings (candy-cane nails). Leukodermic macules are a rarely reported finding. These small macules occur most frequently on the ventral aspect of limbs and trunk. Their onset is prior to puberty. They have been recognized and reported most frequently in individuals with darker skin phototypes. In additional to cutaneous findings, 15%-50% of patients present with oral involvement, including cobblestoning of the oral mucosa, gingival hypertrophy, and sialadenitis. Esophageal involvement with erosions has been described. The severity of oral disease may parallel that of the cutaneous disease. After onset, the disease is lifelong. It may be accentuated or only prominent in the spring and summer, when exposures to heat, perspiration, and ultraviolet (UV) light are increased. Other exacerbating conditions / factors may include trauma, menstruation, and certain drugs (eg, lithium, oral corticosteroids). The lesions of Darier disease may be pruritic, painful, or malodorous. Along with the appearance, these symptoms may lead to significant psychosocial distress. Patients are at an increased risk of bacterial or viral skin infections. -

Tuberous Sclerosis
Tuberous Sclerosis Valerie Ford (Health Education Consultant / Mediator) -------------------------------------------------------------------------------- Definition Tuberous sclerosis is a genetic disorder that causes benign tumors to form in many organs including the brain, eyes, skin, heart, kidneys and lungs. It is characterized by some of the following abnormalities: 1. lesions in the cortex and white matter with seizures (93 per cent) and mental retardation (62 per cent); 2. retina or optic nerve hamartomas (53 per cent) 3. skin fibrous-angiomatous lesions (83 percent) 4. cyst-like areas in phalanges (back) (66 percent) showing evidence of sclerosis 5. renal (kidney and surrounding area) angiomyolipomata (45 to 81 per cent) 6. pit-shaped enamel defects on the teeth Introduction Tuberous sclerosis (also referred to as tuberous sclerosis complex (TSC) to distinguish it from Tourette’s syndrome) is a genetic disease that affects multiple organs. Because of better testing methods, the estimates of those who have the disease have risen dramatically in the last few years. It is now believed that the condition occurs in approximately 1:6000 to 8000 live births and affects approximately 50,000 individuals in the U.S. and more than 1 million worldwide. Males and females seem to be equally affected. While it affects all races, it appears to be uncommon among blacks. It is believed to be inherited through a dominant trait in the 9th or 16th chromosome, with about 86 per cent representing fresh mutations from unaffected parents. It may be diagnosed anytime from birth to adulthood. Over 50 per cent of people with TS have normal intelligence and lead normal lives. -

Multiple, Primary Brain Tumors with Diverse Origins
Published online: 2019-09-02 Case Series Multiple, Primary Brain Tumors with Diverse Origins and Different Localizations: Case Series and Review of the Literature Thara Tunthanathip, Kanet Kanjanapradit1, Sanguansin Ratanalert, Nakornchai Phuenpathom, Thakul Oearsakul, Anukoon Kaewborisutsakul Department of Surgery, Background: Multiple, primary brain tumors with different histological types Neurosurgical Unit, Faculty occurring in the same patient are extremely rare. Several hypotheses have been of Medicine, Prince of Songkla University, proposed, and the pathophysiology of coexisting tumors has long been debated; 1Department of Pathology, however, due to low incidence, standard practices for this scenario are still Abstract Faculty of Medicine, Prince inconclusive. Case Description: The authors describe 6 cases of coexisting of Songkla University, tumors. By conducting a literature research focused on the computed tomography Hat Yai, Songkhla, 90110, (CT) era and patients without prior radiation or phakomatosis. Sixty‑five such Thailand reported cases were identified. In addition, the authors summarize their experience in 6 patients including histopathological features, chronological presentations, outcomes, mortality, and management from their series as well as from previous cases from the reported literature. Conclusion: The coexistence of multiple, primary brain tumors is an interesting condition. Surgical management remains the major treatment; malignant histology has a poor prognostic factor. Keywords: Coexistence of brain tumor, diffuse astrocytoma, glioblastoma, meningioma, multiple primary brain tumor Introduction gender‑specific distribution, the incidence rate of tumor he association of multiple, primary brain tumors of the meninges is higher in females (10.51/100,000) T with different histology occurring simultaneously rather than in males (4.85/100,000), while the in the same patient is an extremely rare condition. -

Symptomatic Splenic Hamartoma: Case Report and Literature Review
Symptomatic Splenic Hamartoma: Case Report and Literature Review ABSTRACT. An 11-year-old girl with low-grade fever, In 1953, Videbaek6 first reported the association of night sweats, thrombocytopenia, and an 8-year history of splenic hamartoma with hematologic disorders in a progressive splenomegaly underwent an elective sple- 30-year-old woman who had onset of symptoms in nectomy. Pathologic diagnosis was multiple splenic childhood. Thirty such symptomatic cases have been hamartoma. The patient’s symptoms resolved after the reported since then. Symptoms reported most fre- splenectomy. Since first described by Rokitansky in quently include pancytopenia, anemia, and throm- 1861, ;140 cases of splenic hamartoma have been de- scribed in the literature. Most of the splenic hamartomas bocytopenia. Less commonly, fever, malaise, and were discovered incidentally. A minority of these lesions weight loss have been reported. Nine cases of symp- were associated with hematologic symptoms such as pan- tomatic splenic hamartoma have been described in cytopenia, anemia, and thrombocytopenia. Only 20 of the pediatric patients. The symptoms observed in the reported cases of splenic hamartoma occurred in pediat- pediatric patients are similar to those in the adult ric patients. However, compared with the adult patients, patient population. In addition, growth retardation, nearly half of these cases in pediatric patients was asso- night sweats, and recurrent infections have been re- ciated with symptoms. Splenectomy and partial splenec- ported in the pediatric patient population.7 Here, we tomy have relieved these symptoms. With advances in report an example of symptomatic splenic hamar- imaging, splenic hamartomas are being discovered with toma in a pediatric patient who had an 8-year span of increasing frequency. -

Tuberous Sclerosis Epilepsy and Psychosis by Adults
Journal of Neurology & Stroke Short Communication Open Access Tuberous sclerosis epilepsy and psychosis by adults Introduction Volume 8 Issue 6 - 2018 The tuberous sclerosis (TS) or tuberous sclerosis complex (TSC) is a multisystem, autosomal dominant disorder by children and adults, Andrej N Ilanković,1 NikolaN Ilanković2 results from mutations in one of two genes, TSC1 (encoding hamartin) 1Universitz Psychiatric Clinic, Dep. of Neuropszchiatrz, Serbia or TSC2 (encoding tuberin). First described in depth by Bourneville in 2General hospital “MEDIGROUP”, Serbia 1880 (Morbus Bourneville, Epiloa).1 TS and TSC often causes disabling neuropsychiatric disorders, Correspondence: Andrej N. Ilanković, MD, PhD, Ass Prof, including epilepsy, mental retardation, autism and psychosis. Universitz Psychiatric Clinic, Dep. of Neuropszchiatrz, Belgrade, Serbia, Pasterova 2, Email Additional major features of the disease include dermatologic manifestations such as facial angiofibromas, renal angiomyolipomas, Received: October 09, 2018 | Published: December 26, 2018 and pulmonary lymphangiomyomatosis. TSC has a wide clinical spectrum of disease, and many patients have minimal signs and symptoms with or without neurologic and psychiatric disability.2 TS is one of “phakomatosis” a group of congenital hereditary developmental anomalies having selective involvement of tissues of ectodermal origin, which develop disseminated glial hamartomas. Examples of phakomatosis are neurofibromatosis, tuberous sclerosis, DERMATOLOGICAL LESIONS Sturge-Weber syndrome, and von Hippel-Lindau disease.”3 Shagrin patch Papula Hypomelanic macula Angiofibroma Figure 2 Typical endocranial (cortical, subcortical and subependymal) lesions by Tuberous sclerosis. The molecular genetics analysis in multigenerational families and positional cloning were used to map both the TSC1 and TSC2 genes 32- 34 . The TSC2 gene, which is located on chromosome 16p13, encodes Figure 1 Typical skin lesion for Tuberous sclerosis. -
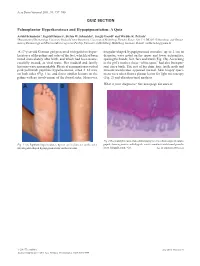
QUIZ SECTION Palmoplantar Hyperkeratoses And
Acta Derm Venereol 2011; 91: 737–740 QUIZ SECTION Palmoplantar Hyperkeratoses and Hypopigmentation: A Quiz Astrid Schmieder1, Ingrid Hausser2, Stefan W. Schneider1, Sergij Goerdt1 and Wiebke K. Peitsch1 1Department of Dermatology, University Medical Centre Mannheim, University of Heidelberg, Theodor-Kutzer-Ufer 1-3, DE-68135 Mannheim, and 2Depart- ment of Dermatology and Electron Microscopy Core Facility, University of Heidelberg, Heidelberg, Germany. E-mail: [email protected] A 17-year-old German girl presented with painless hyper- irregular-shaped hypopigmented macules, up to 2 cm in keratoses of the palms and soles of the feet, which had been diameter, were noted on the upper and lower extremities, noted immediately after birth, and which had been unsuc- sparing the hands, feet, face and trunk (Fig. 1B). According cessfully treated, as viral warts. Her medical and family to the girl’s mother, these “white spots” had also been pre- histories were unremarkable. Physical examination revealed sent since birth. The rest of her skin, hair, teeth, nails and pink-yellowish papulous hyperkeratoses, sized 2–10 mm, mucous membranes appeared normal. Skin biopsy speci- on both soles (Fig. 1A), and fewer similar lesions on the mens were taken from a plantar lesion for light microscopy palms with no involvement of the dorsal sides. Moreover, (Fig. 2) and ultrastructural analysis. What is your diagnosis? See next page for answer. Fig. 2. Haematoxylin eosin-stained skin biopsy specimen from a hyperkeratotic Fig. 1. (A) Papulous hyperkeratoses, up to 1 cm in diameter, on the soles. papule, showing massive orthohyperkeratosis, acanthosis and a broad granular (B) Irregular-shaped hypopigmentations on the forearm. -

Oral Manifestation of Genodermatoses L
Journal of Medicine, Radiology, Pathology & Surgery (2017), 4, 22–27 NARRATIVE REVIEW Oral manifestation of genodermatoses L. Kavya, S. Vandana, Swetha Paulose, Vishwanath Rangdhol, W. John Baliah, T. Dhanraj Department of Oral Medicine and Radiology, Indira Gandhi Institute of Dental Sciences, Pudhucherry, India Keywords Abstract Genodermatoses, mutation, oral Genodermatoses are inherited dermatological disorders associated with the structure manifestation and functions of skin and its appendages. Several genodermatoses presenting with Correspondence multisystem involvement lead to increased morbidity and mortality. Dermatological Dr. L. Kavya, Department of Oral Medicine and diseases, besides including the skin and its supplements may also involve the oral cavity, Radiology, Indira Gandhi Institute of Dental which deserves special attention considering that they may be the only presenting sign Sciences, Pudhucherry, India. of these disorders. The important aspect to be noted about these disorders is the rarity Phone: +91-9442902745. of the conditions and lack of awareness among the population which are the major E-mail: [email protected] drawbacks in the early diagnosis and prompt management of these diseases. This review intends to outline various genodermatoses with their characteristic oral manifestations. Received: 11 April 2017; Accepted: 15 May 2017 doi: 10.15713/ins.jmrps.97 Introduction hence, it is simplified under three classifications based on its distinct features. Genodermatoses or genetic diseases are a group of inherited According to William et al. 2005:[3] skin disorders with a collection of cutaneous and systemic signs 1. Chromosomal and symptoms. In the oral cavity, a wide spectrum of diseases 2. Single gene occurs due to genetic modifications ranging from developmental 3. -

Tuberous Sclerosis Complex the Focus of New Therapies and Continued Research
Tuberous sclerosis complex the focus of new therapies and continued research TSC studies advance understanding of other diseases “Tuberous sclerosis complex is a ‘linchpin’ disease or one in which the genetic pathway is the same as that affecting other diseases and disorders. TSC research is creating breakthroughs in how we treat epilepsy, autism and cancer,” says Joyce Wu, MD, professor of pediatric neurology, director of the UCLA Tuberous Sclerosis Complex Program and a founding member of the Tuberous Sclerosis Complex Clinical Research Consortium. “Every research-hour spent on unlocking the cure to TSC improves our understanding of TSC and so many other Tuberous sclerosis complex (TSC) is an often unrecognized or misdiagnosed diseases as well,” says Dr. genetic disorder that causes benign tumors to develop in multiple organ systems Wu. “The drug everolimus and frequently causes epilepsy. Research being done at UCLA and elsewhere is has been groundbreaking in yielding new understanding of the condition and, in some cases, new treatments. shrinking brain and kidney tumors associated with TSC The UCLA Tuberous Sclerosis Complex Program has been designated by the and is currently in clinical Tuberous Sclerosis Alliance — the TSC national advocacy and research support trials to improve epilepsy and organization — as one of a handful of TSC programs nationwide with the resources neurocognition in TSC even and expertise to address the complexities of this disease and the multiple medical though it is not a seizure or behavioral medication, per se. needs of TSC patients. UCLA’s program includes both clinical care and research and If we can understand how this is one of the largest in the Western United States.