Jack Stapleton, M.D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GB Virus C/Hepatitis G Virus Does Not Induce Expression of P44 Antigen in Chimpanzee Hepatocytes Yohko K
Jpn. J. Infect. Dis., 54, 2001 and skin were removed was purchased on the previous night ongln Of infection was not clear. The food service in a festival of the festival. It was left in the room temperature ovemight. as described in the present case does not requlre regulation The cooking started at 4 0'clock in the momlng by roastlng under the food hyglene law. This incident indicated the the bulk meat by rotation over a gas bumer. The meat was necessity of proper measures for food security in this type of covered by a large steel box during roastlng. It was cut into short temporary food service and regulatory measures in case small pleCeS and served at 10 o'clock in the momlng. Some of possible occurrences of food poISOnlng. noted that some portion of the meat was rare; i.e., it was insufficiently cooked. Laboratory and other epidemiologlCal data will be published ln the outbreak, a total 58 cases were counted. There were in Infectious Agents Surveillance Report, vol. 22 (June, 200 I ). 41 primary infections, 1 1 secondary infections including a We thank the clinical institutions, schools and other institu- case which took place in a nursery school, and 6 cases whose tions fわr their collaboration. Laboratory and Epidemiology Communications GB Virus C/Hepatitis G Virus Does Not Induce Expression of p44 Antigen in Chimpanzee Hepatocytes Yohko K. Shimizu*, Minako Hijikata and Hiroshi Yoshikural Department ofRespiratoyy Diseases, Research Institute, International Medical Center ofJapan, Toyama 1-21-1 Shinjuku-ku, Too,0 162-8655 and JNational Institute ofInfectious Diseases, Toyama 1-23-1, Shinjuku-ku, Tokyo 162-8640 Communicated by Hiroshi Ybshikura (Accepted June 1, 2001) The cytoplasmic antlgen, p44, was orlglnally discovered found chimpanzees whose sera were positive for GBV-C什IGV in hepatocytes of chimpanzees experimentally infected with RNA by RTIPCR. -

Voter Name Residence Address Party Status
06/15/2021 Final Canvass List Page 1 of 289 City/Town: WEST WARWICK Generated By: KERRY NARDOLILLO Voter Name Residence Address Party Status Registration Voter ID Precinct Ward / Council Con Sen Rep Date Dist Dist Dist Dist WEST WARWICK ABDEL, SHEREEN 76 SERVICE RD, WEST WARWICK R A 09/24/2019 38001510307 3808 05-8 2 09 27 ALEXANDRA ABRAHAMSON, ARIANA L 1796 NEW LONDON TPKE Unit U A 02/15/2019 07001456312 3808 05-8 2 09 27 APT C-2, WEST WARWICK ABRANTES, MARK L 95 LONSDALE ST, WEST R A 09/08/2004 38000661071 3808 05-8 2 09 27 WARWICK ABRANTES, RENEE M 95 LONSDALE ST, WEST R A 09/08/2004 38000661072 3808 05-8 2 09 27 WARWICK ACETO, CHERYL A 10 LONGBOW DR, WEST D A 04/28/1999 38000668038 3808 05-8 2 09 27 WARWICK ACETO, LUCA ANTONIO 10 LONGBOW DR, WEST U A 11/25/2014 38001355752 3808 05-8 2 09 27 WARWICK ACKERMAN, PHILIP 14 WHISPER CT, WEST WARWICK U A 01/07/2014 02000602527 3808 05-8 2 09 27 WHEELER ACKERMAN, RENA N 14 WHISPER CT, WEST WARWICK U A 01/08/2014 02000602528 3808 05-8 2 09 27 ACKERMAN, STEVEN P 46 DRAWBRIDGE DR, WEST R A 02/15/2018 06001048151 3808 05-8 2 09 27 WARWICK ACKERMAN, STEVEN P 46 DRAWBRIDGE DR, WEST R A 07/01/1999 38000661079 3808 05-8 2 09 27 WARWICK ACOSTA, DIOMARY O 59 COWESETT AVE Unit APT 73, D A 06/05/2020 28001164253 3808 05-8 2 09 27 WEST WARWICK ACOSTA, JUSTIN 63 COWESETT AVE Unit APT 112, U I 11/07/2018 38001489094 3808 05-8 2 09 27 WEST WARWICK ADAMS, FRANCES M 98 SETIAN LN, WEST WARWICK R A 07/16/1970 38000661090 3808 05-8 2 09 27 ADAMS, SCOTT E 9 BRAMBLE LN, WEST WARWICK U A 07/12/2017 29000597227 3808 -

Prevalence and Clinical Significance of HGV/GBV-C Infection in Patients
Jpn. J. Infect. Dis., 59, 25-30, 2006 Original Article Prevalence and Clinical Significance of HGV/GBV-C Infection in Patients with Chronic Hepatitis B or C Jeng-Fu Yang1,2, Chia-Yen Dai2,3, Wan-Long Chuang2, Wen-Yi Lin1,2, Zu-Yau Lin2, Shinn-Cherng Chen2, Ming-Yuh Hsieh2, Liang-Yen Wang2, Jung-Fa Tsai2, Wen-Yu Chang2 and Ming-Lung Yu1,2* 1Department of Preventive Medicine and 2Department of Medicine, Kaohsiung Medical University Hospital, and 3Department of Occupational Medicine, Kaohsiung Municipal HsiaoKang Hospital, Kaohsiung, Taiwan (Received July 5, 2005. Accepted November 11, 2005) SUMMARY: Hepatitis G virus/GB virus-C (HGV/GBV-C) is a newly identified Flavivirus. Its clinical signifi- cance in chronic hepatitis B and C remains controversial. Infection with HGV/GBV-C was surveyed in 500 blood donors, 130 patients with chronic hepatitis B and 173 with hepatitis C, with chronic liver disease, cirrhosis, and/or hepatocellular carcinoma (HCC). HGV/GBV-C RNA was detected by reverse transcription-polymerase chain reaction. An antibody to HGV/GBV-C’s second envelope protein (anti-E2 Ab) was detected using an enzyme immunoassay. The prevalence of HGV/GBV-C RNA was 3.4% and the exposure rate 10.2% in blood donors. The prevalence of HGV/GBV-C RNA in patients with chronic hepatitis B and hepatitis C was 7.7 and 17.3%, respectively (P = 0.002). The prevalence of the HGV/GBV-C infection in hepatitis B carriers increased with the severity of chronic liver disease and risk of HCC. The age and duration of hepatitis B virus infection were the more important contributing factors. -

Comments Submitted by the Pew Charitable Trusts on Behalf of 6,771 Members of the Public
Comments submitted by The Pew Charitable Trusts on behalf of 6,771 members of the public November 30, 2015 The Honorable Stephen Ostroff, M.D. Acting Commissioner U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 The Honorable Thomas Vilsack Secretary of Agriculture U.S. Department of Agriculture 1400 Independence Avenue, S.W. Washington, D.C. 20250 Director Tom Frieden, M.D. Centers for Disease Control and Prevention 1600 Clifton Rd Atlanta, GA 30333 Re: “Collecting On-Farm Antimicrobial Use and Resistance Data; Public Meeting; Request for Comments,” Docket No. FDA-2015-N-2768 Dear Commissioner Ostroff, Secretary Vilsack, and Director Frieden: FDA took a valuable step in May when it issued a proposed rule to collect species-specific antibiotic sales estimates. Thank you for collaborating with USDA and CDC to obtain public input on possible approaches for collecting additional on-farm antibiotic use and resistance data. To be most useful, on- farm use information should be quantitative and should shed light on why antibiotics are used in food animal production: for treating diseases or for preventing or controlling their spread. This information is essential to enhancing antibiotic stewardship, evaluating whether FDA policies and industry interventions are reducing unnecessary use, and illustrating trends in use and resistance. Your on-farm data collection plan should include the following elements: • Antibiotic class used and medical importance. • Dispensing status. • Purpose of antibiotic use. • Species and animal production class receiving the antibiotic. • Route of administration. Increasing on-farm sampling for antibiotic-resistant bacteria in live animals could help explain where and when antibiotic resistance emerges in the food animal production chain. -

Notre Dame of Mt. Carmel Church
Notre Dame of Mt. Carmel Church 75 Ridgedale Avenue, Cedar Knolls, New Jersey 07927 Parish Office: 973-538-1358 ♦ Fax: 973-538-7403 ♦ www.ndcarmel.com Eucharist Saturday: Sunday: 7:30 a.m., 9:00 a.m., 10:30 a.m. & 12:15 p.m. Monday - Friday: 12:10 p.m. & 5:30 p.m. No weekday 5:30 p.m. Mass from Memorial Day through Labor Day. Holy Days: Vigil 5:30 p.m. Holy Day: 7:15 a.m. 12:10 p.m. & 5:30 p.m. Lent: An additional 7:15 a.m. Mass is celebrated Monday - Friday. Reconciliation Saturday: 12:40 p.m. - 1:15 p.m. or call to see one of the priests. Miraculous Medal Novena & Benediction Monday: 7:30 p.m. Exposition of the Blessed Sacrament Friday: 7:00 a.m. thru 7:00 p.m. Baptism Contact the Parish Secretary to make arrangements. Marriages Contact one of our priests. Sick Calls Contact Fr. Paddy, Fr. Jhon, or Parish Secretary. For Communion for the homebound, Our Parish Vision Notre Dame of Mount Carmel is a Roman Catholic community of faith called to be missionary disciples of Jesus Christ, for the purpose of bringing about the Kingdom. Our Parish Mission Formed by the Word, fed at the Table, and inspired by the Holy Spirit, we INVITE, WELCOME and GUIDE each other into relationship with Christ, to GO OUT and 2019 U O MAKE missionary disciples. “The Word of God will challenge you, Staff inspire you, and give you hope. The Rev. Paddy O’Donovan, Pastor Jean Pankow, Pastoral Associate Ext. -

Precinct Results
Precinct Results Official Results Muskegon County, Michigan Registered Voters 96730 of 148377 = 65.19% Election Night Results General Election Precincts Reporting 75 of 75 = 100.00% 11/3/2020 Run Time 5:55 PM Run Date 11/13/2020 Page 1 Blue Lake Township 1,414 of 2,059 registered voters = 68.67% Straight Party Ticket - Vote for not more than 1 Choice Party Absentee Voting Election Day Voting Total Democratic Party DEM 0 0.00% 307 40.34% 307 40.34% Republican Party REP 0 0.00% 448 58.87% 448 58.87% Libertarian Party LIB 0 0.00% 3 0.39% 3 0.39% US Taxpayers Party UST 0 0.00% 1 0.13% 1 0.13% Working Class Party WCP 0 0.00% 1 0.13% 1 0.13% Green Party GRN 0 0.00% 1 0.13% 1 0.13% Natural Law Party NLP 0 0.00% 0 0.00% 0 0.00% Cast Votes: 0 0.00% 761 100.00% 761 100.00% Overvotes: 0 0 0 Electors of President and Vice-President of the United States - Vote for not more than 1 Choice Party Absentee Voting Election Day Voting Total Joseph R. Biden DEM 0 0.00% 527 37.43% 527 37.43% Kamala D. Harris Donald J. Trump REP 0 0.00% 863 61.29% 863 61.29% Michael R. Pence Jo Jorgensen LIB 0 0.00% 15 1.07% 15 1.07% Jeremy Cohen Don Blankenship UST 0 0.00% 1 0.07% 1 0.07% William Mohr Howie Hawkins GRN 0 0.00% 2 0.14% 2 0.14% Angela Walker Rocky De La Fuente NLP 0 0.00% 0 0.00% 0 0.00% Darcy Richardson Brian T. -
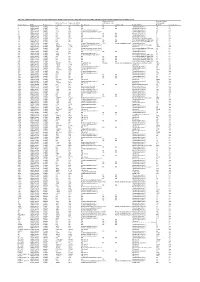
Table S4. Comparison Between the Discvr Results and the BLAST
Table S4. Comparison between the DisCVR results and the BLAST results from the original manuscript for healthy patients and patients with unexplained acute febrile illness Number of k-mers Number of reads Number of k-mers Number of distinct matching the 2nd matching with Sample_Name RUN Patient status matching k-mers matching Top Virus hits hit 2nd Virus Hit Top BLAST hit BLAST Concordance 3 SRR1748140 healthy NA NA NA NA NA Human.adenovirus.C 10 no 4 SRR1748141 healthy NA NA NA NA NA Human.adenovirus.C 65 no 8 SRR1748143 healthy 943 379 Human mastadenovirus C Human.adenovirus.C 65 yes 10 SRR1748147 healthy 1669 133 Human immunodeficiency virus 1 Human.adenovirus.C 26 no 14 SRR1748151 healthy NA NA NA NA NA Human.adenovirus.C 4 no 15 SRR1748152 healthy NA NA NA NA NA Human.adenovirus.C 6 no 108 SRR1748162 healthy NA NA NA NA NA Human.adenovirus.C 6 no 137 SRR1748172 healthy 15343 4714 Human immunodeficiency virus 1 Human.immunodeficiency.virus 314 yes 173 SRR1748173 healthy NA NA NA NA NA Heterosigma.akashiwo.RNA.virus 20 no 245 SRR1748177 healthy NA NA NA NA NA Heterosigma.akashiwo.RNA.virus 17 no 312 SRR1748178 healthy 2843 4 Human T-lymphotropic virus 1 1282 Human mastadenovirus C Human.adenovirus.C 70 no 316 SRR1748179 healthy 22915 5941 Human immunodeficiency virus 1 Human.immunodeficiency.virus 342 yes 319 SRR1748180 healthy 2247683 21201 GB virus C GB.virus.C 20629 yes 325 SRR1748181 healthy 1006 177 Human immunodeficiency virus 1 Heterosigma.akashiwo.RNA.virus 60 no 329 SRR1748182 healthy 1513 124 Human immunodeficiency virus 1 -

1964 Newsletter April
MIAMI PALMETTO HIGH CLASS OF 1964! NEWSLETTER Ron Lieberman, Editor Volume #48, Issue #3 Thad Koch, Associate Editor The Sonesta Hotel, Coconut Grove Site of the 45th Reunion - Miami Palmetto High Class of 1964 CLASSMATE SEARCH, 50TH REUNION, ARCHIVES Thanks to all of you who responded, contributed and enjoyed the updated newsletter format. Your Reunion Committee remains firm in the commitment to locate as many missing Alumni from our Class of 1964. Your help is still needed to find more of our classmates and friends! Please continue to search and provide us with updated information on any missing alums. Also, keep sending your current information, stories or pictures to contribute to the newsletter. Remember, our 50th Reunion will be celebrated in 2014! Soon the Reunion Committee will begin to formulate plans for this signature event. We want all PHS ’64 alumni to take part as we rejoice in past and present friendships and memories. Building our archives is a continuing process. We are looking for more pictures and memorabilia from our school days and especially past reunions. Please send ‘em if you got ‘em! ! PAGE 1 FROM THE EDITOR Greetings to the Class of ’64...we appreciate all the feedback regarding our new look newsletter. The articles and photographs contributed by our fellow classmates make this newsletter what it has become. I have just been given the honor of becoming President of the Rotary Club here in Miami. Bunny and I recently returned home from a fabulous three week trip to Thailand & China, beginning with a week in Bangkok where we attended the 103rd Annual Rotary International Convention. -

GB/Hepatitis G Viruses Likelihood of Secondary Transmission: • Probably Frequent, Based on the Prevalence of Virus in Disease Agent: Blood Donors
APPENDIX 2 GB/Hepatitis G Viruses Likelihood of Secondary Transmission: • Probably frequent, based on the prevalence of virus in Disease Agent: blood donors. • GB viruses (GBV-C/HGV is an acronym for GB virus-C At-Risk Populations: and hepatitis G virus strain variants.) • Blood recipients, injection-drug users, and infants Disease Agent Characteristics: born to infected mothers • Family: Flaviviridae; Genus: Unclassified for GBV-C Vector and Reservoir Involved: • Virion morphology and size: Enveloped, nucleo- capsid of unknown symmetry, 50-100 nm in diameter • Humans • Nucleic acid: Linear, positive-sense, single-stranded Blood Phase: RNA, ~9.4 kb in length • Physicochemical properties: Less stable in CsCl than • Viremic phase can last from weeks to years HCV; other properties not established for these Survival/Persistence in Blood Products: viruses, but, under in vitro conditions, other flavivi- ruses are stable in alkaline environment of pH 8 and • Survives refrigeration are sensitive to treatment with heat, organic solvents, • Inactivated by solvent-detergent and detergents. Transmission by Blood Transfusion: Disease Name: • Well documented in prospective studies • No known disease association Cases/Frequency in Population: Priority Level: • The prevalence of viremia is 1-4%, and antibody • Scientific/Epidemiologic evidence regarding blood prevalence is 3-14% in blood donors. safety: Absent; transmission documented, but no • Prevalence of 10-20% in patients with viral and non- disease associated despite extensive studies viral liver diseases based on antibody and RNA • Public perception and/or regulatory concern regard- • Prevalence of 75-90% in injection-drug users (anti- ing blood safety: Absent body and RNA) • Public concern regarding disease agent: Absent Incubation Period: Background: • Viremia becomes detectable from 2 days to 2 weeks • In the 1960s, serum from a surgeon (GB) with acute postexposure. -

Last Name First Name Amount City State Betat Darlene 50.00
End Citizens United//Let America Vote Action Fund January 2021 Contributions Last Name First Name Amount City State Betat Darlene $ 50.00 Portland OR David Denise $ 300.00 La Jolla CA Etienne-Millot Bernadette $ 7.00 Bethesda MD Evans Mary $ 50.00 Pendleton OR HOGAN Edward $ 10.00 Northampton MA Simon Claudia $ 25.00 Midlothian VA Zuanich Janet $ 10.00 Everett WA Page 1 of 1 End Citizens United//Let America Vote Action Fund February 2021 Contributions Last Name First Name Amount City State Badcock Belinda $ 10,000.00 Greenwich CT Bernard Ellen $ 25,000.00 Harrisville NH Bernard Ed $ 25,000.00 Harrisville NH Blair Clancy $ 100.00 Brooklyn NY Block Peter $ 100.00 Cincinnati OH Boogdanian D $ 7.00 Boston MA Brickner Suzanne $ 50.00 Tulsa OK Burday Rachel $ 25.00 Lagrangeville NY Burke Kathleen $ 1,000.00 Tiburon CA Byrd Nora $ 10.00 Houston TX Carolin Robert $ 25.00 San Diego CA Connolly Taylor $ 5.00 Baltimore MD Cormier Brian $ 500.00 Demastus Vicky $ 5.00 Navarre OH Derig Gene $ 10.00 Anacortes WA DuBois Ann $ 1,000.00 Greenwich CT Etienne-Millot Bernadette $ 7.00 Bethesda MD Fidler Josh $ 25,000.00 Naples FL Fidler Genine $ 25,000.00 Naples FL Gold Eva Kay $ 100.00 Portland OR Goldsborough John $ 25.00 Philadelphia PA Groth Stephen $ 5.00 Laguna Beach CA Groundwater Beth $ 500.00 Breckenridge CO Hanavan William $ 1,000.00 Cleveland Heights OH Heintz Stephen $ 250.00 New York NY Hitchcock Jennifer Megan $ 25.00 Richmond VA Hogan Edward $ 10.00 Northampton MA Hohn John $ 100.00 Winston Salem NC Horn Edward $ 10.00 Yonkers NY Ivanov Pavel -

8.3 Notebook Annotated
Hans L. Tillmann, md Department of Gastroenterology, When Two Infections Hepatology, and Endocrinology Diagnostic Laboratory for Viral and Autoimmune Hepatitis Are Better Than One: Medizinische Hochshule Hannover, Germany The Exceptional Role of Summary by Tim Horn Edited by Douglas Dieterich, md, and GB Virus-C (GBV-C) in Stephen Locarnini, mbbs, phd, frc (path) HIV Disease Reprinted from The prn Notebook® | september 2003 | Dr. James F. Braun, Editor-in-Chief | Tim Horn, Executive Editor. | Published in New York City by the Physicians’ Research Network, Inc.® John Graham Brown, Executive Director | For further information and other articles available online, visit http://www.prn.org | All rights reserved. ©september 2003 when it comes to hiv and hepatitis virus coinfections, the pages fected with hcv (Linnen, 1996). A second virus was isolated in this pa- of The prn Notebook have been filled with numerous reports high- tient, which the Genelabs team called the hepatitis G virus (hgv). lighting the distressing prevalence and negative consequences of both With the discovery of both viruses, researchers set out to deter- hepatitis B virus (hbv) and hepatitis C virus (hcv) in hiv-infected in- mine the full genomic sequences of the isolates. It turned out that dividuals. However, it does not appear that all coinfections are harmful. gbv-c and hgv are closely related isolates of the same virus, with more In fact, some may be associated with a significant survival advantage— than 95% sequence homology (Alter, 1996). It was also determined a theory that has many hiv experts both perplexed and excited. that the genomic organization of these viruses is similar to that of hcv gb virus-c (gbv-c) infection is one such example. -

Period 3 Dream Achievers!
Period 3 Dream Achievers! 3rd Gold Star Dream Achievers 2 S4 RONNIE W MARTIN 322 B1 RAMA VIMAL BHATIA 300 A2 TSAO-YUNG LEE 381 LI CAI 316 C DEEPAK BHATTACHARJEE Period 3 Gold Star Dream Achievers 2 S4 RONNIE W MARTIN TAWAN 310 E 2 X3 LAWRENCE L SANCHEZ DARAKORNNAAYUDTHAYA 4 A3 MARGARET J DUNLEVY 316 C DEEPAK BHATTACHARJEE 322 4 C4 STEPHEN PICCHI RAMA VIMAL BHATIA 20 K2 LINDA M RUGGIERO B1 322 45 TOMMY J WALZ ASHOK KUMAR GUPTA B2 114 M KURT ECKEL 322 F MANESH GAUTAM 300A2 TSAO-YUNG LEE 325 YUKTA PRASAD SHRESTHA 300B2 SHEN-KAO CHOU B1 300 JU-MING CHENG 356 B DO-MOOK KANG G2 381 LI CAI 306 CHAMINDU WIMAN C1 ABEYWICKREMA 385 YUJUAN CHEN 308 386 JIANHUA ZHANG PHENG HOCK DAVID LEE LC 1 FERNANDO DA SILVA MOTA A1 308 BOON HOE LEE B2 Period 3 Silver Star Dream Achievers United States of America, Its Affiliates, Bermuda & The Bahamas 1 CN MORRIS RITZEL 18 L MARK RICE 1 CS WAYMON JOHNSON 19 A PETER CHENG 1 F MOLLY M PENNY 19 C MARILYNN M DANBY 1 G JOHNNY C ANDERSON 19 E KENNETH R COOK 2 A1 FRANK BERTHOLD 19 F STEVEN E NOBLE 2 A2 RODERICK G CHISHOLM III 19 H KENNY S LEE 2 A3 J MICHAEL OLSZEWSKI 20 K1 BARBARA MOODY 2 E2 SUZI SCHNEIDER 20 O WILFRED H ROEHE 2 S1 WALDO E DALCHAU 20 R1 BARBARA CHUCK 2 T3 RAYMOND VIC MORGAN 20 R2 MARIA MARGARITA SIERRA 2 X2 NANCY W VAN ALSTINE 20 W MARK WHITNEY 3 K DARYL E PULTS 21 B CLAROY P HANSON 3 L CAROL SUE THOMPSON 21 C MARC PAQUETTE 4 A1 LEWIS TOM BAUDER 22 C ELIZABETH D HAWKINS 4 A2 WALTER J JUAREZ 23 A EDWARD G HABERLI 4 C1 MARGARET ROBESON 25 A CONSTANCE SUZANNE ROE 4 C5 ANDY ANDERSON 25 B KATHY MORTON 4 L1