Ferroalloy Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vanadium Pentoxide and Other Inorganic Vanadium Compounds
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization. Concise International Chemical Assessment Document 29 VANADIUM PENTOXIDE AND OTHER INORGANIC VANADIUM COMPOUNDS Note that the layout and pagination of this pdf file are not identical to the printed CICAD First draft prepared by Dr M. Costigan and Mr R. Cary, Health and Safety Executive, Liverpool, United Kingdom, and Dr S. Dobson, Centre for Ecology and Hydrology, Huntingdon, United Kingdom Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 2001 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management -

Magnesium Ferrosilicon Alloy and Use Thereof in Manufacture of Nodular Cast Iron
:uropaisches Patentamt 0 1 08 1 07 $ QJll Europeaniuropean Patent Office ® Publication number: B 1 Office)ffice europeen des brevets g) EUROPEAN PATENT SPECIFICATION §) Date of publication of patent specification: 13.01.88 (g) int. CI.4: C 22 C 33/08 |j) Application number: 83901516.1 Date of filing: 28.03.83 ® International application number: PCT/US83/00428 @ International publication number: WO 83/03848 10.11.83 Gazette 83/26 g) MAGNESIUM FERROSILICON ALLOY AND USE THEREOF IN MANUFACTURE OF NODULAR CAST IRON. (§) Priority: 21.04.82 US 370185 (73) Proprietor: SKW ALLOYS INC. 3801 Highland Avenue Niagara Falls New York 14305 (US) (§) Date of publication of application: 16.05.84 Bulletin 84/20 ® Inventor: DREMANN, Charles Earl Buckwalter Road (§) Publication of the grant of the patent: Phoenixville, PA 19460 (US) 13.01.88 Bulletin 88/02 @ Representative: Hale, Stephen Geoffrey et al ® Designated Contracting States: J.Y. & G.W. Johnson Furnival House 14/18 High DE FR GB Holborn London WC1V 6DE (GB) (58) References cited: FR-A-2443 510 GB-A-746406 GB-A- 885 896 GB-A-1 273 319 CD US-A-2 762 705 US-A-2873188 US-A-3 537 842 O US-A-3 703 922 US-A-4004 630 US-A-4224 069 00 Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition shall CL be filed in a written reasoned statement. It shall not be deemed to have been filed until the opposition fee has been LU paid. -

Ferroalloy Quality for Electric Steelmaking with Nonmetallic Inclusion Control
Electrometallurgy UDC 669.168/.785:539.219.1 Ferroalloy Quality for Electric Steelmaking with Nonmetallic Inclusion Control 1 2 2 Gasik M. I. , Panchenko A. I. , Sal`nikov A. S. 1National Metallurgical Academy of Ukraine 4 Gagarin Ave., Dnipropetrovsk, 49600, Ukraine 2JSC “Dneprospetsstal” 81Yuzhnoe Shosse, Zaporizhzhya, 69008, Ukraine Data about quality of ferroalloys used for electric steelmaking and alloys with special properties controlled by specified nonmetallic inclusions according to GOST 801-78 and ASTM E-45 (method A) are generalized and analyzed. Results of investigation of matrix and proeutectoid phases in the structure of industrial carbonaceous ferrochromium with application of electron microscopy and electron probe microanalysis as the main alloying ferroalloy at roller-bearing steel ШХ15СГ-В smelting are presented. Keywords: FERROALLOYS, STANDARDS, MICROSTRUCTURE, MATRIX AND PROEUTECTOID PHASES, NONFERROUS IMPURITY METALS, CARBONACEOUS FERROCHROMIUM, ELECTRON MICROSCOPY, ELECTRON PROBE MICROANALYSIS, NONMETALLIC SILICATE AND SPINDEL INCLUSIONS Introduction Large-scale development and further implementation of electric arc furnaces of large The complex of strength, plastic, anticorrosive unit capacity with electric steel smelting of mass and other properties of alloyed electric steel and application with equipping shops by ladle-furnaces special purpose alloys is defined in many respects and vacuum vessels unedged attention of steel by proeutectoid precipitated phases of endogenetic producers to the important issue of using high origin (oxides, nitrides, sulfides, phosphides) and quality ferroalloys. It is necessary to mention that also impurity non-ferrous metals in the structure of there are companies producing ferroalloys with the rolled (cast) metal. During formation and use of secondary raw materials along with development of electric furnace steelmaking ferroalloy production plants. -

Development of Ni-Free Mn-Stabilised Maraging Steels Using Fe2siti Precipitates
This is a repository copy of Development of Ni-free Mn-stabilised maraging steels using Fe2SiTi precipitates. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/146857/ Version: Accepted Version Article: Knowles, A.J., Gong, P., Rahman, K.M. et al. (3 more authors) (2019) Development of Ni-free Mn-stabilised maraging steels using Fe2SiTi precipitates. Acta Materialia. ISSN 1359-6454 https://doi.org/10.1016/j.actamat.2019.05.034 Article available under the terms of the CC-BY-NC-ND licence (https://creativecommons.org/licenses/by-nc-nd/4.0/). Reuse This article is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs (CC BY-NC-ND) licence. This licence only allows you to download this work and share it with others as long as you credit the authors, but you can’t change the article in any way or use it commercially. More information and the full terms of the licence here: https://creativecommons.org/licenses/ Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Accepted Manuscript Development of Ni-free Mn-stabilised maraging steels using Fe2SiTi precipitates Alexander.J. Knowles, Peng Gong, Khandaker.M. Rahman, W.Mark Rainforth, David Dye, Enrique.I. Galindo-Nava PII: S1359-6454(19)30318-0 DOI: https://doi.org/10.1016/j.actamat.2019.05.034 Reference: AM 15306 To appear in: Acta Materialia Received Date: 24 January 2019 Revised Date: 8 May 2019 Accepted Date: 16 May 2019 Please cite this article as: A.J Knowles, P. -

Arch. Metall. Mater. 63 (2018), 2, 547-553 1. Introduction As An
Arch. Metall. Mater. 63 (2018), 2, 547-553 DOI: 10.24425/118973 LI LEI*,**, ZHANG LIBO*,***,****, DAI LINQING*,***,****#, ZHU HONGBO*,***,****, CHEN GUO*,***,****, PENG JINHUI*,***,****, GUO QIN*,***,**** EFFECTS OF MICROWAVE SINTERING ON PROPERTIES AND MICROSTRUCTURE OF FERROMANGANESE ALLOY POWDERS Microwave sintering process was employed to agglomerate ferromanganese alloy powders. The effects of sintering tempera- ture, holding time and particle size composition on the properties and microstructure of sintering products were investigated. The results was shown that increasing sintering temperature or holding time appropriately is beneficial to increase the compressive strength and volume density. SEM and EDAX analysis shows that the liquid phase formed below the melting point in the sintering process, which leads to densification. XRD patterns indicate that the main reaction during microwave sintering is the decarboniza- tion and carburization of iron carbide phase. The experiment demonstrate that the optimum microwave sintering process condition is 1150°C, 10 min and 50% content of the powders with the size of –75 μm. Keywords: Microwave sintering; Ferromanganese powder; Densification; Liquid phase; Process conditions 1. Introduction in 1999 by Roy et al. [9], the use of microwaves to consolidate a range of particulate metals and alloys has gained growing at- As an important alloy, ferromanganese is widely used as tentions during the past decade. A lot of researches have been deoxidizer and desulfurizer for steelmaking. In addition, it is an performed in microwave sintering ceramics, ferrites and hard essential alloying agent to improve the mechanical properties of metals [10-12]. Comparing with conventional sintering, micro- steel such as hardness, ductility, toughness and anti-wear ability wave sintering have many advantages such as faster heating rate, [1-3]. -
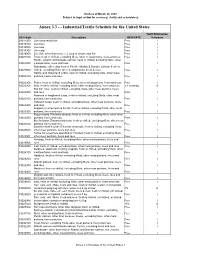
US Schedule for Internet V2
Draft as of March 23, 2007 Subject to legal review for accuracy, clarity and consistency. Annex 3.3 - - Industrial/Textile Schedule for the United States Tariff Elimination US 8 digit Description MFN RATE Schedule 03011000 Live ornamental fish Free I 03019100 Live trout Free I 03019200 Live eels Free I 03019300 Live carp Free I 03019900 Live fish, other than trout, eel, carp or ornamental fish Free I 03021100 Trout, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I Pacific, Atlantic and Danube salmon, fresh or chilled, excluding fillets, other 03021200 meat portions, livers and roes Free I Salmonidae other than trout or Pacific, Atlantic & Danube salmon, fresh or 03021900 chilled, excluding fillets, other meat portions, livers & roes Free I Halibut and Greenland turbot, fresh or chilled, excluding fillets, other meat 03022100 portions, livers and roes Free I 03022200 Plaice, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I 03022300 Sole, fresh or chilled, excluding fillets, other meat portions, livers and roes 1.1 cents/kg A Flat fish, nesi, fresh or chilled, excluding fillets, other meat portions, livers 03022900 and roes Free I Albacore or longfinned tunas, fresh or chilled, excluding fillets, other meat 03023100 portions, livers and roes Free I Yellowfin tunas, fresh or chilled, excluding fillets, other meat portions, livers 03023200 and roes Free I Skipjack or stripe-bellied bonito, fresh or chilled, excluding fillets, other meat 03023300 portions, livers and roes Free -

Economic Impact Analysis (EIA) for the Manganese Ferroalloys RTR
Economic Impact Analysis (EIA) for the Manganese Ferroalloys RTR Final Report EPA-452/R-15-004 May 2015 Economic Impact Analysis (EIA) for the Manganese Ferroalloys RTR By: Larry Sorrels U.S. Environmental Protection Agency Office of Air Quality Planning and Standards (OAQPS) Health and Environmental Impacts Division (HEID) Air Economics Group (AEG) Research Triangle Park, NC 27711 U.S. Environmental Protection Agency Office of Air Quality Planning and Standards Health and Environmental Impacts Division Research Triangle Park, North Carolina CONTENTS Section Page Section 1 Executive Summary ............................................................................................... 1-1 1.1 Analysis Summary ............................................................................................... 1-1 1.2 Organization of this Report.................................................................................. 1-2 Section 2 Introduction ............................................................................................................ 2-3 2.1 Background for Rule ............................................................................................ 2-3 2.1.1 What is this source category and how did the MACT regulate its HAP emissions? ....................................................................................... 2-3 2.1.2 What data collection activities were conducted to support this action?...........................................................................................2-6 2.1.3 Technology Review -

EMEP/EEA Air Pollutant Emission Inventory Guidebook 2019 1
Category Title NFR 2.C.2 Ferroalloys production SNAP 040302 Ferroalloys ISIC Version Guidebook 2019 Coordinator Jeroen Kuenen EMEP/EEA air pollutant emission inventory guidebook 2019 1 2.C.2 Ferroalloys production Contents 1 Overview .......................................................................................................... 3 2 Description of sources .................................................................................... 3 2.1 Process description ...................................................................................................................... 3 2.2 Techniques .................................................................................................................................... 3 2.3 Emissions and controls ............................................................................................................... 4 3 Methods............................................................................................................ 6 3.1 Choice of method ......................................................................................................................... 6 3.2 Tier 1 default approach ............................................................................................................... 6 3.3 Tier 2 technology-specific approach .......................................................................................... 7 3.4 Tier 3 emission modelling and use of facility data ................................................................... 8 4 Data -

The Complete Book on Ferroalloys
The Complete Book on Ferroalloys (Ferro Manganese, Ferro Molybdenum, Ferro Niobium, Ferro Boron, Ferro Titanium, Ferro Tungsten, Ferro Silicon, Ferro Nickel, Ferro Chrome) Author: B.P Bhardwaj Format: Paperback ISBN: 9789381039298 Code: NI258 Pages: 480 Price: Rs. 2,775.00 US$ 250.00 Publisher: NIIR PROJECT CONSULTANCY SERVICES Usually ships within 5 days The Complete Book on Ferroalloys (Ferro Manganese, Ferro Molybdenum, Ferro Niobium, Ferro Boron, Ferro Titanium, Ferro Tungsten, Ferro Silicon, Ferro Nickel, Ferro Chrome) An alloy is a mixture or solid solution composed of metals. Similarly, Ferroalloys are the mixture of Iron with high proportion of other elements like manganese, aluminium or silicon. Alloying improves the physical properties like density, reactivity, Young’s modulus, electrical and thermal conductivity etc. Ferroalloys thus show different properties as mixture of different metals in different proportion exhibit a wide range of properties. Also, Alloying is done to alter the mechanical properties of the base metal, to induce hardness, toughness, ductility etc. The main demand of ferroalloys, nowadays is continuously increasing as the major use of such products are in the field of civil construction; decorative items; automobile; steel industry; electronic appliances. The book provides a wide idea to readers about the usage of appropriate raw material and the treatment involved in the processing of raw material to final produce, safety, uses and properties of raw material involved in the processes. This book concisely presents the core principles and varied details involved in processing of ferroalloys. The work includes detailed coverage of the major products like ferroaluminium, ferrosilicon, ferronickel, ferromolybdenum, ferrotungsten, ferrovanadium, ferromanganese and lesser known minor ferroalloys. -

Critical Evaluation and Thermodynamic Modeling of High Alloy Fe-Mn-Al-Si-C-P System
Critical Evaluation and Thermodynamic Modeling of High Alloy Fe-Mn-Al-Si-C-P System Zhimin You Department of Mining and Materials Engineering McGill University, Montreal, Quebec, Canada August 2020 A thesis submitted to McGill University in partial fulfilment of the requirements of the degree of Doctorate of Philosophy ©Zhimin You, 2020 Dedication To my parents, my brother, and my sister in China Tables of Content Abstract .......................................................................................................................................... 6 Résumé ........................................................................................................................................... 8 Acknowledgements ..................................................................................................................... 10 Preface and Contributions of Authors ...................................................................................... 12 List of Figures .............................................................................................................................. 14 List of Tables ............................................................................................................................... 20 List of Abbreviations and Symbols ........................................................................................... 22 Chapter 1: Introduction ............................................................................................................. 26 1.1 Background ........................................................................................................................ -

Obtainment of Sorbitol on Ferroalloy Promoted Nickel Catalysts Kedelbaev BS*, Korazbekova KU and Kudasova DE M
cie al S nce ri s Kedelbaev et al., J Material Sci Eng 2016, 5:2 te & a E DOI: 10.4172/2169-0022.1000224 M n f g i o n l e a e n r r i n u g o Journal of Material Science & Engineering J ISSN: 2169-0022 Research Article Article OpenOpen Access Access Obtainment of Sorbitol on Ferroalloy Promoted Nickel Catalysts Kedelbaev BS*, Korazbekova KU and Kudasova DE M. Auezov South Kazakhstan State University, Kazakhstan Abstract The systematic study of the activity of the catalyst suspended with ferroalloys additives in catalytic hydrogenation of glucose over a wide variation of process parameters is given in this article. Since nickel catalysts were studied sufficiently, we limited to the data of the phase composition and structure; specific surface of alloys and catalysts based on aluminum-nickel alloys, modified ferroalloys. Results of the study of phase, chemical, particle size distribution and structure of nickel alloys and catalysts have shown that modifying metals affect to the ratio of NiAl3/Ni2Al3 in the alloys, crush crystals, increase catalyst particle size, surface area and large size pore volume and simultaneously increase the micro- and supermicro pore ratio. Highly active, stable and selective catalysts based on nickel for the sorbitol synthesis process was developed. Keywords: Catalysts; Nickel; Autoclave; Hydrogenation; Glucose; As a result of the exothermic reaction, the melting temperature was Sorbitol; Physico-chemical properties; Ferroalloys; Kinetics raised to 1700-1800°С, stirring by the induction field was lasted 3-5 min. The alloy was air-cooled in the graphite molds and was crushed to Introduction grains of 0.25 mm. -

Charcoal As an Alternative Reductant in Ferroalloy Production: a Review
processes Review Charcoal as an Alternative Reductant in Ferroalloy Production: A Review Gerrit Ralf Surup 1,*, Anna Trubetskaya 2 and Merete Tangstad 1 1 Department of Materials Science and Engineering, Norwegian University of Science and Technology, 7491 Trondheim, Norway; [email protected] 2 Department of Chemical Sciences, University of Limerick, V94 T9PX Castletroy, Ireland; [email protected] * Correspondence: [email protected]; Tel.: +47-934-73-184 Received: 12 October 2020; Accepted: 3 November 2020; Published: 9 November 2020 Abstract: This paper provides a fundamental and critical review of biomass application as renewable reductant in integrated ferroalloy reduction process. The basis for the review is based on the current process and product quality requirement that bio-based reductants must fulfill. The characteristics of different feedstocks and suitable pre-treatment and post-treatment technologies for their upgrading are evaluated. The existing literature concerning biomass application in ferroalloy industries is reviewed to fill out the research gaps related to charcoal properties provided by current production technologies and the integration of renewable reductants in the existing industrial infrastructure. This review also provides insights and recommendations to the unresolved challenges related to the charcoal process economics. Several possibilities to integrate the production of bio-based reductants with bio-refineries to lower the cost and increase the total efficiency are given. A comparison of challenges related to energy efficient charcoal production and formation of emissions in classical kiln technologies are discussed to underline the potential of bio-based reductant usage in ferroalloy reduction process. Keywords: charcoal; pyrolysis; bio-based reductant; ferroalloy industry; kiln 1.