Professor Bayley's CV (Pdf)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Masthead 2019
Masthead AngewandteA Journal of the German Chemical Society International Edition Chemie Editorial Board Chair: Annette G. Beck-Sickinger, Universität Leipzig Michael Brands, Bayer (Berlin) Editor: Neville A. Compton Holger Braunschweig, Julius-Maximilians-Universität (Würzburg) Martin Brudermüller, BASF (Ludwigshafen) Deputy Editors: Frank Maaß, Nathalie Weickgenannt Thomas Carell, Ludwig-Maximilians-Universität München Klaus Griesar, Merck (Darmstadt) Editorial Office: Senior Associate Editors: Jens Ackermann, Stefan Grimme, Universität Bonn Jonathan Faiz, Tamaryin Godinho, Hansjörg Grützmacher, Eidgenöss. Techn. Hochschule Zürich Nicole Harrington-Frost, Stephen Horner, (Switzerland) Volker Jacob, Guy Richardson, Rainer Haag, Freie Universität Berlin Rachel Schmidt-Radde, Diane Smith, Christian W. Kohlpaintner, Clariant (Pratteln, Switzerland) Xin Su, Suzanne Tobey Walter Leitner, Rheinisch-Westfälische Technische Hochschule Aachen Senior Web Editor: Mario Müller Wolfgang Parak, Universität Marburg Erwin Reisner, University of Cambridge (UK) Associate Editors: Eric Castro, Wolfgang Schnick, Ludwig-Maximilians-Universität München Arno Knappschneider, Kim Meyer Ferdi Schüth, Max-Planck-Institut für Kohlenforschung (Mülheim) Senior Assistant Editors: Gary Battle, Wolfgang Schuhmann, Ruhr-Universität Bochum Christiane Walter Harald Schwalbe, Johann Wolfgang Goethe-Universität Frankfurt Assistant Editors: Lisa Pecher, Petra Schwille, Max-Planck-Institut für Biochemie (Martinsried) Polina Smirnov, Laura Woodward Armido Studer, Westfälische -

CHAD A. MIRKIN, PH.D. Northwestern University
CHAD A. MIRKIN, PH.D. Northwestern University, Department of Chemistry 2145 Sheridan Road, Evanston, IL 60208-3113 Phone: 847-491-2907 Fax: 847-467-5123 Email: [email protected] Education 1991 NSF Postdoctoral Fellow in Chemistry, Massachusetts Institute of Technology, Cambridge, MA 1989 Ph.D. in Inorganic & Organic Chemistry, The Pennsylvania State University, State College, PA 1986 B.S. in Chemistry (Phi Beta Kappa), Dickinson College, Carlisle, PA Professional Experience 2008-present Director, International Institute for Nanotechnology; George B. Rathmann Professor of Chemistry, Medicine, Materials Science & Engineering, Biomedical Engineering, Chemical & Biological Engineering, Northwestern University 2004-2008 Director, International Institute for Nanotechnology; George B. Rathmann Professor of Chemistry, Medicine, and Materials Science & Engineering, Northwestern University 2000-2004 Director, Center for Nanofabrication and Molecular Self-Assembly and George B. Rathmann Professor of Chemistry, Northwestern University 1997-2000 Charles E. and Emma H. Morrison Professor of Chemistry, Northwestern University 1995-1997 Associate Professor, Department of Chemistry, Northwestern University 1991-1995 Assistant Professor, Department of Chemistry, Northwestern University Awards and Honors (selected, over 230 national and international total) 2020 ACS Division of Colloid and Surface Science Award for Outstanding Achievement in Nanoscience; AAAS Philip Hauge Abelson Award 2019 Kabiller Prize in Nanoscience and Nanomedicine; SCI Perkin -

CURRICULUM VITAE CHAO-JUN LI, Ph.D
CURRICULUM VITAE CHAO-JUN LI, Ph.D. Department of Chemistry Tel: 514-398-8457 McGill University, Montreal, Quebec H3A 0B8 Canada E-mail: [email protected] ACADEMIC EXPERIENCE: 1/2009- present E. B. EDDY PROFESSOR OF CHEMISTRY, Department of Chemistry, McGill University 7/2003- present PROFESSOR OF CHEMISTRY, Department of Chemistry, McGill University 7/2003- present CANADA RESEARCH CHAIR (Tier I) (in GREEN/ORGANIC CHEMISTRY) 5/2012-2018 DIRECTOR, NSERC CREATE CENTER FOR GREEN CHEMISTRY TRAINING 5/2009-present Co-DIRECTOR, FQRNT CENTER FOR GREEN CHEMISTRYAND CATALYSIS 5/2009-present DIRECTOR, CFI INFRASTR. FOR GREEN CHEMISTRY AND GREEN CHEMICALS 1/2008-2016 Co-CHAIR, CANADIAN GREEN CHEMISTRYAND ENGINEERING NETWORK 5/2005-1/2008 COORDINATOR, CANADIAN GREEN CHEMISTRY NETWORK 7/2000-7/2003 PROFESSOR OF CHEMISTRY, Department of Chemistry, Tulane University, New Orleans, USA 2002 (Fall) VISITING PROFESSOR, Department of Chemistry, University of California, Berkeley, USA 7/1998-7/2000 ASSOCIATE PROFESSOR, Department of Chemistry, Tulane University, New Orleans, USA 7/1994-7/1998 ASSISTANT PROFESSOR, Department of Chemistry, Tulane University, New Orleans, USA EDUCATION: 1992-1994 NSERC POST-DOCTORAL FELLOW (Organic Synthesis, Stanford University) Advisor: Professor B. M. Trost 1989-1992 Ph.D. (Organic Chemistry, McGill University) Advisors: Professors T. H. Chan and D. N. Harpp 1985-1988 M.S. (Organic Synthesis, Chinese Academy of Science) Advisor: Professor T. H. Chan (McGill University) 1979-1983 B.S. (Chemistry, Zhengzhou University, China) -

Publishing Catalogue 2016 Royal Society of Chemistry RSC Gold Everything You Need in One Sparkling Online Package
Publishing Catalogue 2016 Royal Society of Chemistry RSC Gold Everything you need in one sparkling online package RSC Gold is: • Balanced and complete – stay connected to all the key content within the chemical sciences spectrum • International – authors and editorial boards from over one hundred countries contribute, ensuring a truly global perspective • Flexible – we offer tiered pricing options based on your institution’s size and scope, no matter how big or small RSC Gold includes: • A wide-ranging list of Royal Society of Chemistry Caltech’s RSC Gold journal, database and magazine content package subscription has • Our Journals Archive lease 1841-2007 been a very welcome development ... I am very • A bonus eBook series appreciative of the general excellence of articles in the • The option to continually upgrade and expand your RSC research journals, subscription with new journals and our latest published evidenced by strong impact factors and material increases in local download statistics. There’s also Gold for Gold… Dana L Roth Caltech Library, US Purdue University, USA Gold for Gold voucher codes can be used to publish new chemical science papers in any Royal Society of Chemistry journal via the Gold open access option. Each voucher code can be used to publish one paper free of charge, and is a good way to ensure maximum visibility for your institution’s research. Find out more – contact your Account Manager or email [email protected] Registered charity number: 207890 Royal Society of Chemistry | Publishing | www.rsc.org/publishing Contacts For contact details please see the Regional Representatives and Agents list on page 40. -

Publishing Catalogue 2016 Royal Society of Chemistry Pick and Choose Build Your Own Ebook Collection
Publishing Catalogue 2016 Royal Society of Chemistry Pick and Choose Build your own eBook Collection No set collections – just your pick of key chemical science titles from the Royal Society of Chemistry’s eBook portfolio. Good for your library. Perfect for your budget. It only takes £1,000 to build topic sets like these: 1 3 Set one: Analytical Science 2 Set three: Medicinal Chemistry & Biomolecular Science Set two: Energy & Environmental Science If you don’t have access to our Librarian Portal, it’s simple to register for an account and create your collection. Visit http://rsc.li/pickandchoose to get started. Registered charity number: 207890 Royal Society of Chemistry | Publishing | www.rsc.org/publishing Contacts For contact details please see the Regional Representatives and Agents list on page 40. Contents Sections Page Page RSC eBook Collection 8 Nanoscale 19 The Historical Collection 24 Nanoscale Horizons 20 Collections for 2016 38-39 Natural Product Reports 20 Regional Representatives and Agents 40 New Journal of Chemistry 21 Organic & Biomolecular Chemistry 21 Journals Organic Chemistry Frontiers 27 Analyst 4 Photochemical & Photobiological Sciences 22 Analytical Methods 4 Physical Chemistry Chemical Physics (PCCP) 22 Biomaterials Science 5 Polymer Chemistry 23 Catalysis Science & Technology 5 Reaction Chemistry & Engineering 23 Chemical Communications 6 RSC Advances 25 Chemical Science 6 Soft Matter 25 Chemical Society Reviews 7 Toxicology Research 26 Chemistry Education Research and Practice 7 CrystEngComm 9 Literature Updating -

RSC Gold 2015 Flyer.Pdf
RSC Gold Want access to full content from the world’s leading chemistry society? Including regular new material and an Archive dating back to 1841? Caltech’s RSC Gold Plus voucher codes to publish package subscription has been a very Open Access (OA) free of charge? welcome development ... I am very appreciative of the RSC Gold is the Royal Society of Chemistry’s general excellence of articles in the RSC premium package comprising 41 international research journals, evidenced by strong journals, literature updating services and impact factors and magazines that will meet the needs of all your increases in local download statistics. end-users. And the accompanying Gold for Gold Dana L. Roth OA voucher codes ensure maximum visibility for Chemistry Librarian your institution’s quality research. Caltech, USA Take a look inside to see exactly what you get www.rsc.org/gold RSC Gold includes a wealth of quality RSC journal, database and magazine content that is all available online. Journals Natural Product Reports Analyst New Journal of Chemistry Analytical Methods Organic & Biomolecular Chemistry Biomaterials Science Photochemical & Photobiological Sciences Catalysis Science & Technology Physical Chemistry Chemical Physics (PCCP) Chemical Communications Polymer Chemistry Chemical Science* RSC Advances Chemical Society Reviews Soft Matter CrystEngComm Toxicology Research Dalton Transactions Energy & Environmental Science B a c k fi l e Environmental Science: Nano** RSC Journals Archive 1841-2007 lease Environmental Science: Processes & Impacts -

FORMER GRADUATE STUDENTS Name Degree and Thesis Title Postdoc Institution Current Position and Location Contact Information Year Dr
FORMER GRADUATE STUDENTS Name Degree and Thesis Title Postdoc Institution Current Position and Location Contact Information Year Dr. Lee D. Arnold 1987, Ph.D. Serine β-Lactones in Syntheses of Amino Acids None President & CEO of Discovery Elucidations [email protected] Dr. Hanaa Assil 1989, M.Sc. Solid Supports for Azodicarboxylates in Mitsunobu Reactions n/a Unknown Unknown and Synthesis of Amino Acids Dr. Karine Auclair 1999, Ph.D. Biosynthetic Studies on the Polyketide Lovastatin: Enzyme- Prof. P. Ortiz De Montellano, Department Assoc. Professor, Department of Chemistry, McGill University, [email protected] Catalyzed Diels-Alder Reactions of Pharmaceutical Chemistry, University of Montreal, Que., Canada California at San Francisco, USA Dr. Jennifer Blunston (nee Caplan) 2001, Ph.D. Diaminopimelic Acid Analogues as Inhibitors of Enzymes Prof. D. Waisman, Departments of Stay-at-home mom, raising son Rhys. Previously Jennifer [email protected] Involved in Bacterial Lysine Biosynthesis Biochemistry and Oncology, University of Blunston was Assistant Professor of Chemistry, St. Mary's Calgary, Canada University College, Calgary, AB, Canada Dr. Marc Boudreau 2007, Ph.D. Nucleoside Dicarboxylates as Mimics of Diphosphates and Prof. Hagan Bayley, Oxford University Postdoc with Professor Shahriar Mobashery, University of Notre [email protected] Disulfide Bond Replacement in Pediocin PA1 Dame Dr. Doug Burr 2006, Ph.D. Studies of the in vitro Activity of Lovastatin Nonaketide Prof. David Sherman, Dept. Medicinal Research scientist at Perkin Elmer, Boston, Massachusetts [email protected] Synthase Chemistry, University of Michigan, Ann Arbor, MI Dr. Hengmiao (Henry) Cheng 1992, Ph.D. Mechanism and Inhibition of Peptidylglycine α-Hydroxlating Prof. E.J. Corey, Department of Chemistry, Senior Research Investigator, Pfizer Global Research and [email protected] Monooxygenase Harvard University Development, Pfizer Inc., Groton, CT, USA Dr. -

Bios and Abstracts of RSC Speakers
Colin Bain graduated in Natural Sciences from Cambridge before crossing the Atlantic to undertake his PhD at Harvard with Prof. George Whitesides. He then returned to Cambridge as a Royal Society Fellow, working with Dr. Paul Davies to set up the UK’s first sum-frequency spectrometer. In 1991 he moved to Oxford as a University Lecturer and Fellow of Magdalen College. In 2005 he took up his current position of Professor of Chemistry at Durham University. His research interests lie principally in the chemistry and physics of interfaces, with applications in detergents, personal care products, printing, spraying and lubrication. Prof. Bain has received the Harrison, Corday-Morgan and Tilden Prizes from the Royal Society of Chemistry as well as awards in Japan and Australia. In 2005 he was honoured to deliver the annual McBain Lecture at the National Chemical Laboratory in Pune. He has collaborated with the Indian Institute of Science in Bangalore for a number of years and currently holds a joint UKIERI award with the IISc. Professor Colin Bain Since 2008, he has been a Director of the Institute of Advanced Study at Department of Chemistry Durham – an institute that seeks to promote interdisciplinary dialogue Durham University and research collaborations across the humanities, sciences and social South Road sciences. He has been actively involved in many aspects of industry- Durham DH1 3LE academia interactions, through research collaborations with industry, as U.K. a Director of the Oxford Science Park and as a scientific advisor to a [email protected] Phone (+44) 191 334 2138 venture capital partnership. -

Publishing Price List 2016
Publishing Price List 2016 Royal Society of Chemistry Collections for 2016 RSC GOLD INCLUDES: Key Royal Society of Chemistry online Price on application ONLINE ONLY† journal, database and magazine content, plus a EMAIL [email protected] book series. or contact your Account Manager PRICES JOURNALS ARCHIVE ONLINE ONLY† RSC Journals Archive Outright Purchase (1841 – 2007) • £41,097 • $72,615 RSC Journals Archive Outright Purchase (2005 – 2007) • £4,867 • $8,271 RSC Journals Archive Lease (1841 – 2007) • £7,408 • $13,133 PRICES RSC Journals Archive Hosting Fee • £819 • $1,340 THE HISTORICAL COLLECTION INCLUDES: Price on application ONLINE ONLY† • Society Publications (1949 – 2012) EMAIL [email protected] • Society Minutes (1841 – 1966) or contact your Account Manager • Historical Papers (1505 – 1991) PRICES CORE CHEMISTRY COLLECTION INCLUDES: • Chemical Communications • Dalton Transactions • Journal of Materials Chemistry A, B & C • New Journal of Chemistry PRINT & ONLINE† ONLINE ONLY† • Organic & Biomolecular Chemistry • • • Physical Chemistry Chemical Physics £19,685 £18,701 • RSC Advances (online only) • $36,814 • $34,973 PRICES GENERAL CHEMISTRY COLLECTION INCLUDES: • Chemical Communications • Chemical Society Reviews PRINT & ONLINE† ONLINE ONLY† • Chemistry World • £8,360 • £7,942 • New Journal of Chemistry PRICES • $13,685 • $13,244 • RSC Advances (online only) ANALYTICAL SCIENCE COLLECTION INCLUDES: • Analyst • Analytical Abstracts (online only) • Analytical Methods PRINT & ONLINE† ONLINE ONLY† • Environmental Science: Processes & Impacts • -

Successfully Transitioning the World's Largest Chemistry Subscription
Insights – 30(1), March 2017 Transitioning RSC Advances to gold OA | Emma K Wilson and Jamie Humphrey Successfully transitioning the world’s largest chemistry subscription journal to a gold open access publication In 2016 the Royal Society of Chemistry announced that from January 2017 it would convert RSC Advances, the world’s largest chemistry journal, from a subscription journal to a gold open access journal. This article gives some background to the decision to convert, and provides an update on how the conversion to the new access model has gone during the first three months following conversion. The effect of the conversion on article submissions, including scientific topics covered and the geographical representation of the submitted articles, is also discussed. Introduction As a learned society and the UK’s professional body for those working in the chemical sciences, everything we do at the Royal Society of Chemistry is focused on our mission – to advance excellence in the chemical sciences. One of our four charter objectives is ‘to foster and encourage the growth and application of such science by the dissemination of chemical knowledge’. To this end, our publishing programme started in 1841 and has flourished into a successful, growing and dynamic international programme encompassing a portfolio of journals, books, chemical databases and our magazine Chemistry World. EMMA K WILSON Director of Publishing Our goal is to operate in a sustainable way to meet the publishing needs of the chemistry Royal Society of community and our customers while continuing to reinvest all surplus back into the Chemistry chemistry community. That surplus supports conferences and events, grants and bursaries, awards and prizes. -

GUT RSC Journals List
SCHEDULE A Publisher Content Section A Customer has access to the electronic versions of the following journals via an External route: Access Post - Copyright Journals E-ISSN years cancellation Owner* during Term access Analyst 1364-5528 2008-2018 2012-2018 RSC Analytical Methods 1 1759-9679 2009-2018 2012-2018 RSC Annual Reports on the Progress of Chemistry, A 1460-4760 2008-2013 2012-2013 RSC B 1460 4779 2008-2013 2012-2013 RSC C 1460-4787 2008-2013 2012-2013 RSC Biomaterials Science 1 2047-4849 2013-2018 2016-2018 RSC Catalysis Science & Technology 1 2044-4761 2011-2018 2013-2018 RSC Chemical Communications 1364-548X 2008-2018 2012-2018 RSC Chemical Science 1, 2 2041-6539 2010-2014 2012-2014 RSC Chemical Society Reviews 1460-4744 2008-2018 2012-2018 RSC Chemistry World 1749-5318 2012-2016 2012-2016 RSC CrystEngComm 1466-8033 2008-2018 2012-2018 RSC Dalton Transactions 1477-9234 2008-2018 2012-2018 RSC Education in Chemistry 1749-5326 2012-2016 2012-2016 RSC Energy & Environmental Science 1 1754-5706 2008-2018 2012-2018 RSC Environmental Science: Nano 1 2051-8161 2014-2018 2016-2018 RSC Environmental Science: Processes & Impacts including 2050-7895 2013-2018 2013-2018 RSC Journal of Environmental Monitoring (1464-0333) 2008-2012 2012 Environmental Science: Water Research & Technology 1 2053-1419 2015-2018 2017-2018 RSC Faraday Discussions 1364-5498 2008-2018 2012-2018 RSC Food & Function 1 2042-650X 2010-2018 2012-2018 RSC Green Chemistry 1463-9270 2008-2018 2012-2018 RSC Inorganic Chemistry Frontiers 1 2052-1553 2014-2018 2017-2018 -
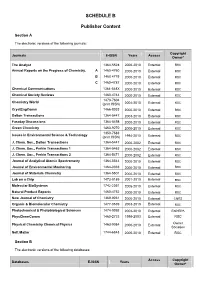
SCHEDULE B Publisher Content
SCHEDULE B Publisher Content Section A The electronic versions of the following journals: Copyright Journals E-ISSN Years Access Owner* The Analyst 1364-5528 2000-2010 External RSC Annual Reports on the Progress of Chemistry, A 1460-4760 2000-2010 External RSC B 1460 4779 2000-2010 External RSC C 1460-4787 2000-2010 External RSC Chemical Communications 1364-548X 2000-2010 External RSC Chemical Society Reviews 1460-4744 2000-2010 External RSC 1473-7604 Chemistry World (print ISSN) 2004-2010 External RSC CrystEngComm 1466-8033 2000-2010 External RSC Dalton Transactions 1364-5447 2003-2010 External RSC Faraday Discussions 1364-5498 2000-2010 External RSC Green Chemistry 1463-9270 2000-2010 External RSC 1350-7583 Issues in Environmental Science & Technology (print ISSN) 1994-2010 External RSC J. Chem. Soc., Dalton Transactions 1364-5447 2000-2002 External RSC J. Chem. Soc., Perkin Transactions 1 1364-5463 2000-2002 External RSC J. Chem. Soc., Perkin Transactions 2 1364-5471 2000-2002 External RSC Journal of Analytical Atomic Spectrometry 1364-5544 2000-2010 External RSC Journal of Environmental Monitoring 1464-0333 2000-2010 External RSC Journal of Materials Chemistry 1364-5501 2000-2010 External RSC Lab on a Chip 1473-0189 2001-2010 External RSC Molecular BioSystems 1742-2051 2005-2010 External RSC Natural Product Reports 1460-4752 2000-2010 External RSC New Journal of Chemistry 1369-9261 2000-2010 External CNRS Organic & Biomolecular Chemistry 1477-0539 2003-2010 External RSC Photochemical & Photobiological Sciences 1474-9092 2002-2010