Historical Biogeography and Interspecific Mtdna Introgression In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Premium Wagyu History Nature
Matsusaka City Guide Nature Premium Wagyu History Matsusaka City Matsusaka is located in Mie Prefecture, near the center of Japan. With the sea to the east and mountains in the west, Matsusaka’s gorgeous natural scenery is a joy in every season. World-famous Matsusaka Cattle are raised right here in the city. Matsusaka, where history and culture blend Matsusaka City Tourist into peoples’ everyday lives, is the perfect 1 2 Matsusaka Cotton Center Information Center 松阪もめん手織りセンター place to enjoy with a leisurely stroll around 豪商のまち松阪 観光交流センター This center works to hand down knowledge about the traditional On the first floor you can get information on sightseeing handicraft Matsusaka Cotton. Fabric, kimono, and accessories on town. and places to eat in Matsusaka. There are also many sale all feature a beautiful indigo striped pattern, and make excellent souvenirs and special products of Matsusaka for sale. The souvenirs. Also popular with customers is the authentic Edo-period second floor introduces the history of Matsusaka through a loom, which you can use to hand-weave the fabric yourself. video playing in the theatre room (with English subtitles) and an exhibit of informational panels. This facility is a great place to start exploring Matsusaka City. Matsusaka IC 0 100 200 300m Matsusaka Agricultural Park Bell Farm 12 4 Former Ozu Residence Motoori Norinaga Matsusaka City (Matsusaka Merchant Museum) Memorial Museum/ Cultural Asset Center Former Hasegawa Residence 10 Suzu-no-Ya "Haniwa Museum" 3 旧小津清左衛門家 8 本居宣長記念館・鈴屋 旧長谷川治郎兵衛家 ATM This is the former residence of Ozu Seizaemon, a 166 The Hasegawa family made its fortune as cotton Castle Guard Matsusaka Castle successful merchant who traded paper and cotton on a Residences 7 merchants in Edo (now Tokyo). -
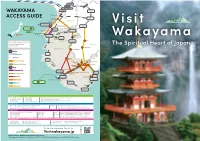
Wakayama Access Guide
WAKAYAMA ACCESS GUIDE Izumisano WAKAYAMA Pref. Buses between Koyasan and Ryujin Onsen run from April 1st to November 30th. Nanki-Shirahama Airport Tokyo 1h 20min TRAVEL BY TRAIN Kansai WIDE Area Pass 5 days validity https://www.westjr.co.jp/global/en/ticket/pass/kansai_wide/ Ise-Kumano Area Pass 5 days validity http://touristpass.jp/en/ise_kumano/ Kansai Thru Pass 2 days validity http://www.surutto.com/tickets/kansai_thru_english.html Kansai One Pass Rechargeable travel card https://kansaionepass.com/en/ TRAVEL BY AIR Haneda Airport (Tokyo) to Kansai International Airport (KIX) 1h 15min http://www.haneda-airport.jp/inter/en/ Haneda Airport (Tokyo) to Nanki-Shirahama Airport 1h 20min https://visitwakayama.jp/plan-your-trip/shirahama-airport/ JAL Explorer Pass (Aordable domestic fares with this pass from Japan Airlines) https://www.world.jal.co.jp/world/en/japan_explorer_pass/lp/ TRAVEL BY BUS Kansai International Airport (KIX) to Wakayama Limousine Bus 50min https://www.kansai-airport.or.jp/en/touristinfo/wakayama.html Kyoto to Shirahama Express Bus 4h https://visitwakayama.jp/itineraries/meiko-bus-osaka-kyoto/ Osaka to Shirahama Express Bus 3h 30min https://visitwakayama.jp/itineraries/meiko-bus-osaka-kyoto/ Tokyo to Shirahama Express Bus 12h https://visitwakayama.jp/itineraries/meiko-bus-tokyo/ Koyasan to Kumano (Daily between April 1 and November 30) Koyasan & Kumano Access Bus 4h 30min https://visitwakayama.jp/good-to-know/koyasan-kumano-bus/ TRAVEL BY CAR Times Car Rental https://www.timescar-rental.com/ Nissan Rent a Car https://nissan-rentacar.com/english/ -

The Chiba Bank, Ltd. Integrated Report 2020
The Chiba Bank, Ltd. Integrated Report 2020 The Chiba Bank, Ltd. 1-2, Chiba-minato, Chuo-ku, Chiba-shi, Chiba 260-8720, Japan Integrated Report Phone: 81-43-245-1111 https://www.chibabank.co.jp/ 005_9326487912009.indd 1-3 2020/09/10 11:20:10 Introduction Our Philosophy Corporate Data The Chiba Bank, Ltd. As of March 31, 2020 Aiming to enhance “customer Principal Shareholders experience” as a partner to customers The ten largest shareholders of the Bank and their respective shareholdings as of March 31, 2020 were as follows: Number of Shares Percentage of Total (in thousands)*1 Shares Issued*2 (%) and regional communities The Master Trust Bank of Japan, Ltd. (Trust Account) 56,139 7.55 Japan Trustee Services Bank, Ltd. (Trust Account) 35,615 4.79 Nippon Life Insurance Company 26,870 3.61 The Dai-ichi Life Insurance Company, Limited 26,230 3.53 Sompo Japan Nipponkoa Insurance Inc.*3 18,537 2.49 Meiji Yasuda Life Insurance Company 18,291 2.46 SUMITOMO LIFE INSURANCE COMPANY 17,842 2.40 MUFG Bank, Ltd. 17,707 2.38 STATE STREET BANK AND TRUST COMPANY 505223 14,576 1.96 Japan Trustee Services Bank, Ltd. (Trust Account 5) 13,406 1.80 Management Policy Excluded from the figures above are 72,709 thousand treasury shares in the name of the Chiba Bank, Ltd. (Excludes one thousand shares which, although registered in the name of the Chiba Bank, Ltd. on the shareholder list, are not actually owned by the Bank.) As a regional financial institution based in Chiba Prefecture, Chiba Bank Group recognizes that *1 Rounded down to the nearest thousand *2 Rounded down to two decimal places its mission is to “contribute to the sustainable development of regional economies through the *3 The trade name of Sompo Japan Nipponkoa Insurance Inc. -

Natural Selection in the Origin
Natural selection in the origin: how does selection act on snails’ shell colour in the source of a diversified population? Shun Ito1, Takahiro Hirano1, Satoshi Chiba1, and Junji Konuma2 1Tohoku University 2Toho University June 7, 2021 Abstract The mechanisms of adaptive radiation with phenotypic diversification and further adaptive speciation have been becoming clearer through a number of studies. Natural selection is one of the primary factors that contribute to these mechanisms. It has been demonstrated that divergent natural selection acts on a certain trait in adaptive radiation. However, it is not often known how natural selection acts on the source of a diversified population, although it has been detected in phylogenetic studies. Our study demonstrates how selection acts on a trait in a source population of diversified population using the Japanese land snail Euhadra peliomphala simodae. This snail’s shell colour has diversified due to disruptive selection after migration from the mainland to islands. We used trail-camera traps to identify the cause of natural selection on both the mainland and an island. We then conducted a mark-recapture experiment on the mainland to detect natural selection and compare the shape and strength of it to previous study in an island. In total, we captured and marked around 1,700 snails, and some of them were preyed on by an unknown predator. The trail-camera traps showed that the predator is the large Japanese field mouse Apodemus speciosus, but this predation did not correlate with shell colour. A Bayesian approach showed that the stabilising selection from factors other than predation acted on shell colour. -

Summary of Family Membership and Gender by Club MBR0018 As of November, 2008
Summary of Family Membership and Gender by Club MBR0018 as of November, 2008 Club Fam. Unit Fam. Unit Club Ttl. Club Ttl. District Number Club Name HH's 1/2 Dues Females Male TOTAL District 334 C 24664 ARAIMACHI L C 0 0 0 27 27 District 334 C 24665 ATAMI 0 0 0 22 22 District 334 C 24671 FUJI GAKUNAN 3 3 0 65 65 District 334 C 24672 FUJIEDA 0 0 0 45 45 District 334 C 24673 FUJINOMIYA 0 0 3 68 71 District 334 C 24674 FUKUROI 0 0 1 59 60 District 334 C 24676 GOTENBA 0 0 0 97 97 District 334 C 24677 HAINAN 0 0 1 49 50 District 334 C 24678 HAMAMATSU 0 0 3 132 135 District 334 C 24679 HAMAKITA 0 0 1 40 41 District 334 C 24682 HIGASHIIZU 0 0 1 21 22 District 334 C 24683 OKUHAMANAKO L C 0 0 0 20 20 District 334 C 24693 ITO 0 0 0 42 42 District 334 C 24694 IWATA 0 0 4 65 69 District 334 C 24695 KAKEGAWA 0 0 1 45 46 District 334 C 24697 KANBARA 0 0 0 44 44 District 334 C 24702 KAWANE 0 0 0 53 53 District 334 C 24708 KOSAI 0 0 2 53 55 District 334 C 24720 MISHIMA 0 0 0 34 34 District 334 C 24722 MORIMACHI L C 0 0 3 41 44 District 334 C 24723 NAGAIZUMI 0 0 0 11 11 District 334 C 24747 NISHIIZU 0 0 0 19 19 District 334 C 24749 NUMAZU 0 0 0 70 70 District 334 C 24750 NUMAZU SENBON 0 0 0 43 43 District 334 C 24753 OHITO 0 0 0 28 28 District 334 C 24761 OYAMA 0 0 0 27 27 District 334 C 24765 SHIMIZUCHO 0 0 0 15 15 District 334 C 24766 SHIBAKAWA 0 0 0 19 19 District 334 C 24767 SHIZUOKA TACHIBANA 0 0 0 52 52 District 334 C 24768 SHUZENJI 0 0 0 30 30 District 334 C 24770 SHIMADA 0 0 2 71 73 District 334 C 24771 SHIMIZU HAGOROMO 0 0 0 33 33 District 334 -

The Ultrastructure of Spermatozoa and Spermiogenesis in Pyramidellid Gastropods, and Its Systematic Importance John M
HELGOLANDER MEERESUNTERSUCHUNGEN Helgol~inder Meeresunters. 42,303-318 (1988) The ultrastructure of spermatozoa and spermiogenesis in pyramidellid gastropods, and its systematic importance John M. Healy School of Biological Sciences (Zoology, A08), University of Sydney; 2006, New South Wales, Australia ABSTRACT: Ultrastructural observations on spermiogenesis and spermatozoa of selected pyramidellid gastropods (species of Turbonilla, ~gulina, Cingufina and Hinemoa) are presented. During spermatid development, the condensing nucleus becomes initially anterio-posteriorly com- pressed or sometimes cup-shaped. Concurrently, the acrosomal complex attaches to an electron- dense layer at the presumptive anterior pole of the nucleus, while at the opposite (posterior) pole of the nucleus a shallow invagination is formed to accommodate the centriolar derivative. Midpiece formation begins soon after these events have taken place, and involves the following processes: (1) the wrapping of individual mitochondria around the axoneme/coarse fibre complex; (2) later internal metamorphosis resulting in replacement of cristae by paracrystalline layers which envelope the matrix material; and (3) formation of a glycogen-filled helix within the mitochondrial derivative (via a secondary wrapping of mitochondria). Advanced stages of nuclear condensation {elongation, transformation of fibres into lamellae, subsequent compaction) and midpiece formation proceed within a microtubular sheath ('manchette'). Pyramidellid spermatozoa consist of an acrosomal complex (round -

Lions Clubs International Club Membership Register Summary the Clubs and Membership Figures Reflect Changes As of March 2005
LIONS CLUBS INTERNATIONAL CLUB MEMBERSHIP REGISTER SUMMARY THE CLUBS AND MEMBERSHIP FIGURES REFLECT CHANGES AS OF MARCH 2005 CLUB CLUB LAST MMR FCL YR MEMBERSHI P CHANGES TOTAL DIST IDENT NBR CLUB NAME STATUS RPT DATE OB NEW RENST TRANS DROPS NETCG MEMBERS 5494 025243 ABIKO 333 C 4 03-2005 14 3 0 0 -2 1 15 5494 025249 ASAHI 333 C 4 03-2005 80 1 0 0 -1 0 80 5494 025254 BOSHUASAI L C 333 C 4 03-2005 15 1 0 0 -2 -1 14 5494 025255 BOSHU SHIRAHAMA L C 333 C 4 03-2005 20 1 0 0 -2 -1 19 5494 025257 CHIBA 333 C 4 03-2005 59 2 0 0 -3 -1 58 5494 025258 CHIBA CHUO 333 C 4 03-2005 30 0 0 0 0 0 30 5494 025259 CHIBA ECHO 333 C 4 03-2005 33 0 1 0 -2 -1 32 5494 025260 CHIBA KEIYO 333 C 4 03-2005 29 1 0 0 0 1 30 5494 025261 CHOSHI 333 C 4 03-2005 46 6 0 0 0 6 52 5494 025266 FUNABASHI 333 C 4 03-2005 20 2 0 0 -1 1 21 5494 025267 FUNABASHI CHUO 333 C 4 02-2005 58 17 0 0 -3 14 72 5494 025268 FUNABASHI HIGASHI 333 C 4 03-2005 27 5 0 0 -2 3 30 5494 025269 FUTTSU 333 C 4 03-2005 29 0 0 0 -2 -2 27 5494 025276 ICHIKAWA 333 C 4 03-2005 33 3 0 0 -2 1 34 5494 025277 ICHIHARA MINAMI 333 C 4 02-2005 28 2 0 0 -2 0 28 5494 025278 ICHIKAWA HIGASHI 333 C 4 03-2005 19 2 0 0 0 2 21 5494 025279 IIOKA 333 C 4 03-2005 36 2 0 0 -1 1 37 5494 025282 ICHIHARA 333 C 4 03-2005 27 1 0 0 -1 0 27 5494 025292 KAMAGAYA 333 C 4 03-2005 30 2 0 0 0 2 32 5494 025297 KAMOGAWA 333 C 4 03-2005 42 3 1 0 -4 0 42 5494 025299 KASHIWA 333 C 4 03-2005 48 0 0 0 -1 -1 47 5494 025302 BOSO KATSUURA L C 333 C 4 03-2005 67 3 1 0 -3 1 68 5494 025303 KOZAKI 333 C 4 03-2005 34 0 0 0 -2 -2 32 5494 025307 KAZUSA -

Launch of “Hikari-De-Net 10G,” Fastest Internet Services in Shizuoka Prefecture, Using Own Fiber-Optic Lines
July 12, 2017 To whom it may concern TOKAI Holdings Corporation Katsuhiko Tokita, President & CEO (Code No. 3167 Tokyo Stock Exchange First Section) Launch of “Hikari-de-net 10G,” Fastest Internet Services in Shizuoka Prefecture, Using Own Fiber-optic Lines TOKAI Cable Network Corporation (Headquarters: Numazu-shi, Shizuoka; President & CEO: Yasuhiro Fukuda), a wholly-owned subsidiary of TOKAI Holdings Corporation (hereinafter “the Company”) engaging in the Group’s CATV business, will start offering “Hikari-de-net 10G,” Internet connection services offering a maximum downstream speed of 10Gbps*1, the fastest*2 in Shizuoka Prefecture, on July 14 as described in the attachment. The Company engages in the CATV business in five prefectures across Japan (Shizuoka, Kanagawa, Chiba, Nagano, and Okayama Prefectures), providing services to a total of 740,000 customers including broadcasting services to 510,000 customers and communication services to 230,000 customers. There has been a sharp increase in communication traffic for consumer services due to the recent increase in devices with communication functions. In order to respond to the situation, the Company has developed an environment to expand the data transmission capacity by changing its head-end equipment*3 from GE-PON to 10G-EPON, realizing the fastest Internet connection services in Shizuoka Prefecture. By providing multiple services, including Internet connection services offering a maximum downstream speed of 10Gbps ahead of other companies as well as broadcasting services and Hikari Denwa, the Company will strive to increase ARPU (Average Revenue Per User) and promote the acquisition of new customers while enhancing services for the existing customers with the use of smartphone set discount*4. -

Izu Peninsula Geopark Promotion Council
Contents A. Identification of the Area ........................................................................................................................................................... 1 A.1 Name of the Proposed Geopark ........................................................................................................................................... 1 A.2 Location of the Proposed Geopark ....................................................................................................................................... 1 A.3 Surface Area, Physical and Human Geographical Characteristics ....................................................................................... 1 A.3.1 Physical Geographical Characteristics .......................................................................................................................... 1 A.3.2 Human Geographical Charactersitics ........................................................................................................................... 3 A.4 Organization in charge and Management Structure ............................................................................................................. 5 A.4.1 Izu Peninsula Geopark Promotion Council ................................................................................................................... 5 A.4.2 Structure of the Management Organization .................................................................................................................. 6 A.4.3 Supporting Units/ Members -

Annual Report
2016 Paper Carton Recycling Paper Carton Recycle Loop Annual Report Dairy product and paper container manufacturers collaborate to recycle paper containers such as milk cartons to preserve the environment. Committee for Milk Container Environmental Issues Nyugyo Kaikan, Kudan Kita 1-14-19, Chiyoda Ku, Tokyo 102-0073 Phone: +81(0)3-3264-3903, Fax: +81(0)3-3261-9176 Recycling rather than trashing http://www.yokankyo.jp makes you feel good. A message to members of paper carton collection associations Please contact schools, local governments, public facilities, retail stores, and welfare organizations to gain their cooperation with regard to installing collection boxes. At the same time, please set up a system to enable regular collection of paper cartons. If you do not know where paper cartons are collected, please contact the section in charge of the local government/public administration. Contact the following for more information: Japan Milk Carton Recycling Association (JAMRA) Phone: +81(0)3-3360-1098, Fax: +81(0)3-3360-7090 Higashi Nakano 4-6-7-201, Nakano Ku, Tokyo 164-0003 Committee for Milk Container Environmental Issues Published: January 2016 Chairperson’s message Action Plan to Improve the Paper Carton Collection Rate By setting the goal to achieve a collection rate of more than 2. Promoting domestic paper carton collection 50% in fiscal 2015, the Committee for Milk Container ①Creation of opportunities for collection Environmental Issues (COMCEI) aims to enhance the paper ②Promotion of collection of 500 ml and 200 ml paper carton collection rate. cartons (smaller than the standard 1000 ml cartons) Nearly five years have passed since the Great East Japan Earthquake. -

Endemic Land Snail Fauna (Mollusca) on a Remote Peninsula in the Ogasawara Archipelago, Northwestern Pacific1
Endemic Land Snail Fauna (Mollusca) on a Remote Peninsula in the Ogasawara Archipelago, Northwestern Pacific1 Satoshi Chiba2,3, Angus Davison,4 and Hideaki Mori3 Abstract: Historically, the Ogasawara Archipelago harbored more than 90 na- tive land snail species, 90% of which were endemic. Unfortunately, about 40% of the species have already gone extinct across the entire archipelago. On Haha- jima, the second-largest island and the one on which the greatest number of species was recorded, more than 50% of species are thought to have been lost. We report here the results of a recent survey of the snails of a remote peninsula, Higashizaki, on the eastern coast of Hahajima. Although the peninsula is small (@0.3 km2) and only part is covered by forest (<0.1 km2), we found 12 land snail species, all of which are endemic to Ogasawara. Among these species, five had been thought to already be extinct on Hahajima, including Ogasawarana yoshi- warana and Hirasea acutissima. Of the former, there has been no record since its original description in 1902. Except for the much larger island of Anijima and the main part of Hahajima, no single region on the Ogasawara Archipelago maintains as great a number of native land snail species. It is probable that the land snail fauna of the Higashizaki Peninsula is exceptionally well preserved be- cause of a lack of anthropogenic disturbance and introduced species. In some circumstances, even an extremely small area can be an important and effective refuge for threatened land snail faunas. The native land snail fauna of the Pacific one such example: of 95 recorded species, islands is one of the most seriously endan- more than 90% are endemic (Kuroda 1930, gered faunas in the world (e.g., Murray et al. -

1,500 New Students Begin Journey by JIU Times
Produced by × Vol. 23 SPRING 2018 1,500 new students begin journey by JIU Times Josai International University welcomed about 1,500 new stu- dents, including foreign people from 18 countries, in a matricula- tion ceremony at its Togane Cam- pus in Chiba Prefecture, on April 2. JIU, a comprehensive university with eight faculties and 10 depart- ments, focuses on international education and is engaged in ex- change programs with many uni- versities and schools in Asia, Eu- rope, particularly Eastern Europe, pay attention to the world events and the Americas. Of its approxi- and news and participate actively mately 6,000 students, 1,438 were in local community activities to ex- non-Japanese from 24 countries perience practical learning.” last year. He also encouraged new Japa- In the matriculation ceremony, nese students to participate in ex- which took place at the Sports Cul- change programs with overseas ture Center, JIU President Kenji schools because studying different Sugibayashi greeted the new stu- cultures is a good experience. dents, as well as other students, Sugibayashi also stressed ad- teachers and special guests, in- vancement of high technology and cluding Togane Mayor Naoharu the importance of learning amid Shiga. the current global environment. “JIU has three campuses in To- “With globalization and rapid gane, Awa (Chiba Prefecture) and advancement of science and tech- Kioicho (Tokyo’s Chiyoda Ward). nology, existing things disappear changes and I believe people can dents, Josai University Educational ing, “What I will be tomorrow is We have undergraduate and grad- or become completely different make innovation happen by learn- Corporation Chancellor Akira Ue- the result of what I think and do uate programs, as well as senior things.