Peripheral Arterial Disease Guidelines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Deep Venous Thrombosis in Postoperative Vascular Surgical Patients: a Frequent Finding Without Prophylaxis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Deep venous thrombosis in postoperative vascular surgical patients: A frequent finding without prophylaxis Maureen Hollyoak, MBBS, MMedSci, FRACS, Peter Woodruff, MBBS, FRCS(Edin), MCh, FRACS, FACS, Michael Muller, MBBS, FRACS, Nicholas Daunt, MBBS, FRCR(Eng), FRACR, and Paula Weir, BSc, DMU (Vasc), Brisbane, Australia Purpose: The place of routine perioperative thromboprophylaxis for vascular surgical patients remains controversial, because the incidence of postoperative deep venous thrombosis (DVT) is said to be quite low. This study was designed to measure the incidence of lower limb DVT after vascular surgical procedures. Methods: All consenting, consecutive patients who came to a metropolitan veterans hospital for abdominal or lower-limb arterial surgery were studied. Clinical and operative data were recorded. Lower-limb color flow duplex scans were per- formed before and after surgery. Results: Fifty patients, age 75 ± 1 (mean ± SEM) years, were studied. Abdominal procedures were performed on 22 patients, and lower-limb procedures were performed on 28 patients. A postoperative DVT was noted in 14 patients (32%), 9 patients (41%) in the abdominal surgical group and 5 patients (18%) in the lower-limb group. Calf DVTs were four times more common than femoropopliteal DVTs. Conclusion: The incidence of postoperative lower-limb DVTs in this cohort of vascular surgical patients was high. The small size of the study population precludes generalized recommendations, but the results indicate an urgent need for definitive investigation. (J Vasc Surg 2001;34:656-60.) Most surgical patients should receive perioperative inability to walk 50 m. -

Sciatic Nerve Block: a Useful Procedure for Diabetic Foot Surgery
Sciatic nerve block: A useful procedure for diabetic foot surgery Florian Heid, Robert Kampka, Gunther Pestel, Tim Piepho Article points 1. Patients requiring surgery The range of comorbidities experienced by people who require for the management of lower-limb surgery to manage diabetic foot disease are many. diabetic foot ulceration These comorbidites make the undertaken of general anaesthesia have a range of comorbidites that increase both difficult and places them at high risk of complications during the risks associated with surgery or in the immediate post-operative period. In this article the general anaesthesia. authors present a description of a peripheral nerve block procedure 2. The authors describe the use of sciatic nerve block as an alternative to general anaesthesia in patients undergoing lower- as an alternative to general limb surgery. Two case reports are also presented. anaesthesia. 3. A range of benefits to the eople with diabetic foot disease by electric nerve stimulation. The electrical patient are associated with regularly have severe comorbidities nerve stimulator (we use Stimuplex HNS eliminating the need for ® general anaesthesia in the P resulting in a high-risk profile for 11 ; Braun, Germany) produces an electrical presence of comorbidites. anaesthesia (American Diabetes Association, current that depolarises the nerve membrane 4. The authors suggest that 2003; Prompers et al, 2007). General and causes contraction of the effector those involved in diabetic anaesthesia and neuroaxial blockade (e.g. muscles of the relevant area. This confirms foot surgery consider spinal anaesthesia) may impair hemodynamic the proximity of the needle to the nerve. the use of nerve block stability. -

Blood Vessel Replacement: 50 Years of Development and Tissue Engineering Paradigms in Vascular Surgery
Physiol. Res. 58 (Suppl. 2): S119-S139, 2009 MINIREVIEW Blood Vessel Replacement: 50 years of Development and Tissue Engineering Paradigms in Vascular Surgery J. CHLUPÁČ1,2,3, E. FILOVÁ1,2, L. BAČÁKOVÁ1,2 1Center for Cardiovascular Research, 2Department of Growth and Differentiation of Cell Populations, Institute of Physiology, Academy of Sciences of the Czech Republic, 3Transplant Surgery Clinic, Institute for Clinical and Experimental Medicine, Prague, Czech Republic Received September 1, 2009 Accepted October 30, 2009 Summary Introduction The gold standard material in bypass surgery of blood vessels remains the patient’s own artery or vein. However, this material Atherosclerosis accounts for almost one half of may be unavailable, or may suffer vein graft disease. Currently all deaths in Europe (Stehouwer et al. 2009). Although available vascular prostheses, namely polyethylene terephthalate advanced pharmacological and minimally-invasive (PET, Dacron) and expanded polytetrafluoroethylene (ePTFE), techniques offer a growing therapy option (Met et al. perform well as large-caliber replacements, but their long-term 2008), a surgical bypass of blood vessels on the heart or patency is discouraging in small-caliber applications (<6 mm), on a lower extremity remains the procedure of choice in a such as in coronary, crural or microvessel surgery. This failure is number of patients (Fig. 1) (Guyton 2006, Norgren et al. mainly a result of an unfavorable healing process with surface 2007). This approach is also more cost-effective, and in thrombogenicity, due to lack of endothelial cells and anastomotic particular preserves the quality of the patient’s life better intimal hyperplasia caused by hemodynamic disturbances. An than primary amputation of a limb (Cheshire et al. -

Open Versus Endovascular Revascularization of Below-Knee Arteries in Patients with End-Stage Renal Disease and Critical Limb
Original Article Vascular and Endovascular Surgery 2018, Vol. 52(8) 613-620 ª The Author(s) 2018 Open Versus Endovascular Revascularization Article reuse guidelines: sagepub.com/journals-permissions of Below-Knee Arteries in Patients With DOI: 10.1177/1538574418789036 End-Stage Renal Disease and Critical journals.sagepub.com/home/ves Limb Ischemia Alexander Meyer, MD1 , Anne Schilling1, Magdalena Kott1, Ulrich Rother, MD1, Werner Lang, MD1, and Susanne Regus, MD1 Abstract Background: Evaluation of below-the-knee open revascularization (OR) versus endovascular revascularization (EVT) in patients with end-stage renal disease and critical limb ischemia (CLI) was performed. Patients and Methods: Seventy-seven dialysis patients with CLI and infrapopliteal involvement from 2007 to 2017 were included. Thirty-five patients received OR and 42 patients were treated with EVT. Survival, amputation-free survival (AFS) and wound-healing were evaluated. Furthermore, both groups were analyzed for differences as to anatomic (lesion length, runoff, pedal arch classification) and clinical (VSG risk score, WIfI score) characteristics. Results: Amputation-free survival (1-year AFS: OR 54.5% vs 47.6% in EVT, 2-year AFS OR 38.3% vs 23.9% EVT, P ¼ .201) did not significantly differ between OR and EVT nor did the wound healing rate (29% OR vs 31% EVT, P ¼ .532). Overall survival was noticeably poor (1-year survival: 66.7% in OR and 49% in EVT, 2-year survival OR 47.4% vs EVT 27.7%; P ¼ .088); evaluation of peripheral runoff (Rutherford score 6.9 OR vs 7.1 EVT, P ¼ .499) and pedal arch classification as well as WIfI or VSG risk score (9.8 OR vs 9.6 EVT, P ¼ .673) could not detect significant differences as to both the groups. -

Femoro-Supragenicular Popliteal Bypass with a Bridging Stent Graft
Korean J Thorac Cardiovasc Surg 2017;50:371-377 □ CLINICAL RESEARCH □ ISSN: 2233-601X (Print) ISSN: 2093-6516 (Online) https://doi.org/10.5090/kjtcs.2017.50.5.371 Femoro-Supragenicular Popliteal Bypass with a Bridging Stent Graft in a Diffusely Diseased Distal Target Popliteal Artery: Alternative to Below-Knee Popliteal Polytetrafluoroethylene Bypass 1 2 3 Joung Hun Byun, M.D. , Tae Gyu Kim, M.D. , Yun Gyu Song, M.D., Ph.D. 1 Department of Thoracic and Cardiovascular Surgery, Gyeongsang National University Changwon Hospital, Departments of 2 3 Radiation Oncology and Radiology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine Background: Lesions in distal target arteries hinder surgical bypass procedures in patients with peripheral ar- terial occlusive disease. Methods: Between April 2012 and October 2015, 16 patients (18 limbs) with life- style-limiting claudication (n=12) or chronic critical limb ischemia (n=6) underwent femoral–above-knee (AK) polytetrafluoroethylene (PTFE) bypass grafts with a bridging stent graft placement between the distal target popliteal artery and the PTFE graft. Ring-supported PTFE grafts were used in all patients with no available vein for graft material. Follow-up evaluations assessed clinical symptoms, the ankle-brachial index, ultrasono- graphic imaging and/or computed tomography angiography, the primary patency rate, and complications. Results: All procedures were successful. The mean follow-up was 12.6 months (range, 11 to 14 months), and there were no major complications. The median baseline ankle-brachial index of 0.4 (range, 0.2 to 0.55) sig- nificantly increased to 0.8 (range, 0.5 to 1.0) at 12 months (p<0.01). -
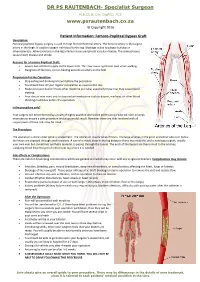
Femoro Popliteal Bypass Graft
DR PS RAUTENBACH- Specialist Surgeon M.B.Ch.B; DA; DipPEC; FCS www.psrautenbach.co.za © Copyright 2016 Patient Information: Femoro-Popliteal Bypass Graft Description: Femoral popliteal bypass surgery is used to treat blocked femoral artery. The femoral artery is the largest artery in the thigh. It supplies oxygen-rich blood to the leg. Blockage is due to plaque buildup or atherosclerosis. Atherosclerosis in the leg arteries causes peripheral vascular disease. The same process causes heart disease and stroke. Reasons for a Femoro-Popliteal Graft: Severe lack of blood supply to the lower limb. This may cause significant pain when walking. Gangrene of the toes, or non-healing wounds or ulcers on the feet. Preparation for the Operation: Stop eating and drinking 6 hours before the procedure. You should take all your regular medication as usual on the day. Make sure your doctor knows what medicine you take, especially those that may cause blood clotting. Your doctor may want you to stop certain medication such as disprin, warfarin, or other blood thinning medicines before the operation. Is the procedure safe? Your surgery will be performed by a team of highly qualified and skilled professionals who will take all steps necessary to ensure a safe procedure and a successful result. However there are risks involved with all surgery even if these risks may be small. The Procedure: The operation is done under general anaesthetic. The operation usually takes 3 hours. The large arteries in the groin and either above or below the knees are exposed through small incisions. A tunnel is made deep in the leg between these two incisions, and a new bypass graft, usually your own vein but sometimes synthetic material, is passes through the tunnel. -

Outcomes of Diabetic Foot Patients Following Peripheral Vascular
International Journal of Medical Science and Clinical Inventions 4(4): 2823-2826, 2017 DOI:10.18535/ijmsci/v4i4.2 ICV 2015: 52.82 e-ISSN:2348-991X, p-ISSN: 2454-9576 © 2017, IJMSCI Research Article Outcomes of Diabetic Foot Patients Following Peripheral Vascular Bypass Surgery Amit Kumar C Jain1, Subramanyam SG2, Viswanath S3 1 Assistant professor, Department of surgery, St John’s Medical College, Bangalore, India 2 Professor, Department of surgery, St John’s Medical College, Bangalore, India 3 Associate professor, Department of surgery, St John’s Medical College, Bangalore, India CORRESPONDING AUTHOR: DR AMIT KUMAR C JAIN ABSTRACT: Aim: The aim of this study was to analyse the outcomes of peripheral vascular disease and the bypass procedure done in patients with diabetic foot problems. Methods and materials: A cross sectional descriptive retrospective analysis was carried in department of Surgery at St John’s medical college, Bangalore, India. The study period was for 3 years from February 2012 to Jan 2015. Results: A total of 17 patients had undergone open peripheral bypass surgery procedure for diabetic foot problems during this period. There were 13 males [76.5%] and 4 females [23.5%]. 11 patients [64.7%] had history of smoking. 6 patients [35.3%] had diabetes of less than 1 year duration. 7 patients [41.1%] had presented with dry gangrene. 5 patients [29.41%] had associated foot infection. 9 patients [52.9%] had undergone open bypass surgery only without any amputation done on them. The most common surgical procedure performed was femoro-popliteal bypass surgery [76.47%]. Conclusion: The recent trend shows an increase in endovascular procedure over bypass procedure which is decreasing over years. -

Technology Assessment
Technology Assessment Horizon Scan of Invasive Interventions for Lower Extremity Peripheral Artery Disease and Systematic Review of Studies Comparing Stent Placement to Other Interventions Technology Assessment Program October 10, 2008 Prepared for: Agency for Healthcare Research and Quality 540 Gaither Road Rockville, Maryland 20850 1 Horizon Scan of Invasive Interventions for Lower Extremity Peripheral Artery Disease and Systematic Review of Studies Comparing Stent Placement to Other Interventions Technology Assessment Report Project ID: ARTS0407 October 10, 2008 Tufts Evidence-based Practice Center, Boston MA Ethan Balk, MD MPH M. Soledad Cepeda, MD PhD Stanley Ip, MD Thomas Trikalinos, MD PhD Thomas O’Donnell, MD This report is based on research conducted by the Tufts Evidence-based Practice Center under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. HHSA-290-2007-10055-1-EPC3). The findings and conclusions in this document are those of the authors who are responsible for its contents; the findings and conclusions do not necessarily represent the views of AHRQ. No statement in this article should be construed as an official position of the Agency for Healthcare Research and Quality or of the U.S. Department of Health and Human Services. 2 The information in this report is intended to help health care decision-makers; patients and clinicians, health system leaders, and policymakers, make well- informed decisions and thereby improve the quality of health care services. This report is not intended to be a substitute for the application of clinical judgment. Decisions concerning the provision of clinical care should consider this report in the same way as any medical reference and in conjunction with all other pertinent information, i.e., in the context of available resources and circumstances presented by individual patients. -

Axillo-Bipopliteal Bypass Application in Critical Leg Ischemia
AXILLO-BIPOPLITEAL BYPASS APPLICATION IN CRITICAL LEG ISCHEMIA We performed an extended extra-anatomic bypass to salvage the both lower extremities in a diabetic and obes woman who had critical ischemia in her both lower extremities. Patient also had several abdominal operations. The patient did not have a good arterial vas- CELALETTİN GÜNAY, MD culature as inflow or outflow conduit in the right femoral region and ALPER UÇAK, MD* she also had femoro-popliteal bypass which was occluded on the left FARUK CİNGÖZ, MD lower limb. Firstly, trombectomy was performed to the occluded graft. HAKAN BİNGÖL, MD Secondly, left axillofemoral, thirdly, left to right cross-over, finally, ERKAN KURALAY, MD femoro-popliteal bypasses were performed to make an axillo- bipopliteal bypass functionally. The case will be evaluated under the literature knowledge. Key words: Extra-anatomic, femoropopliteal bypass. INTRODUCTION rterial reconstruction with extra-anatomic bypass surgey (Ex-AB) is performed for complex vascular pathologies From: which can not be managed with classical conventional Gulhane Military Medical Academy, Amethods. The Ex-AB concept was firstly suggested by Freeman Department of Cardiovascular Surgery, Etlik Ankara Turkey and Leeds (1) in 1949 and three years later the first femoro- * GATA Haydarpasa Training Hospital, femoral bypass was performed. Blaisdell FW and Hall AD (2) Department of Cardiovascular Surgery, were the first who performed a unilateral axillo-femoral bypass Kadikoy Istanbul Turkey (Ax-FB) by using prothesis of Dacron graft, but Sauvage LR and Woods SJ (3) were the first who introduced a less traumatic sur- gery by performing axillo-bifemoral bypass (Ax-bFB). Salvaging of more than one extremity with a synthetic graft was performed by Smith (4) in 1977 and Veith (4) in 1978. -

V02 Femoro-Popliteal Bypass Surgery
V02 Femoro-Popliteal Bypass Surgery Expires end of October 2016 Issued December 2015 You can get information locally from East Lancashire Hospitals NHS Trust main switchboard on 01254 263 555. You can also contact: ............................................................................................................................... ..................... ............................................................................................................................... ..................... ............................................................................................................................... ..................... Get more information and references at www.aboutmyhealth.org Tell us how useful you found this document at www.patientfeedback.org www.rcseng.ac.uk www.rcsed.ac.uk www.asgbi.org.uk www.pre-op.org What is atherosclerosis? Atherosclerosis is a disease affecting the arteries. Abnormal fatty material (called atheroma) coats the inside of an artery, causing it to narrow or harden. The amount of blood flowing through the artery is reduced. When the arteries to your legs are affected, you will have pain in the back of your legs when you Femoral artery walk. If the narrowing gets more severe, you may have pain even when you are resting. If the blood supply continues to get worse, you may develop ulcers or even gangrene of your toes or feet. Your surgeon has recommended a femoro-popliteal bypass operation. However, it is your decision to go ahead with the operation or not. This document will -
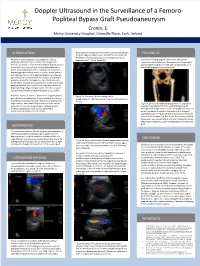
Popliteal Bypass Graft Pseudoaneurysm Printing: Cronin, E
Doppler Ultrasound in the Surveillance of a Femoro- Popliteal Bypass Graft Pseudoaneurysm Printing: Cronin, E. [Replace the following names and titles with those of the actual contributors: Dorena Paschke, PhD1; David Alexander, PhD2; Jeff Hay, RN, BSN, MHA3, and Pilar Pinilla, MD4 1[Add affiliation for first contributor], 2[Add affiliation for second contributor], 3[Add affiliation for third contributor], 4[Add affiliation for fourth contributor] This poster is 48” wide by 36” high. Mercy University Hospital, Grenville Place, Cork, Ireland. It’s designed to be printed on a large INTRODUCTION 9 months later, the patient returned for surveillance of both FOLLOW UP Customizing the Content: the graft and pseudoaneurysm. The graft remained patent. However, the pseudoaneurysm had increased in size, as The placeholders in this Peripheral arterial disease is associated with serious demonstrated in Figure 2a and 2b. 2 months following surgical intervention, the patient morbidity and mortality and involves the progressive returned to the Accident and Emergency department with formatted for you. stenosis, occlusion or aneurysmal dilatation of the aorta and pain and absent pulses in his left calf. Consequently, a CT its non-coronary, non-intracranial branches (Sibley et al., lower limb angiogram was performed. placeholders to add text, or click an 2016). Revascularisation of the limb plays a critical role in the management of the disease. In relation to the femoro- icon to add a table, chart, SmartArt popliteal segment, femoro-popliteal bypass is an effective graphic, picture or multimedia file. intervention for advanced occlusive disease, of which the preferred conduit is the saphenous vein (Vartanian and Conte, 2015). -
Neuropathic Pain After Femoropopliteal Bypass Surgery
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Neuropathic pain after femoropopliteal bypass surgery A. Greiner, MD,a B. Rantner, MD,a K. Greiner, MD,b F. Kronenberg, MD,d M. Schocke, MD,c B. Neuhauser, MD,a J. Bodner, MD,a G. Fraedrich, MD,a and A. Schlager, MD,b Innsbruck, Austria Objective: This retrospective study was performed to investigate prolonged postoperative pain in the area of the proximal or distal scar or the bypass tunnel after femoropopliteal bypass surgery to treat symptomatic peripheral arterial disease. Patients and Methods: Ninety-three patients with peripheral arterial disease who underwent femoropopliteal bypass surgery between January 2000 and December 2002 were included in the study. The short-form McGill Pain Question- naire was used to score pain. Ultrasound examination of the soft tissue around the graft was performed to exclude other pathologic conditions responsible for pain, such as inflammatory processes, perigraft reactions, swollen lymph nodes, and hematomas. Results: Pain in at least one scar existed in 22 patients on average 13.9 ؎ 9.8 months after surgery. In 10 patients pain existed simultaneously along the inguinal scar and the above-knee or below-knee scar. Pain along the bypass tunnel was experienced by seven patients. Most patients had mild to moderate pain. The mean numeric ranking score of pain severity ,in patients with pain was 4.2 ؎ 2.3. The occurrence of prolonged postoperative pain was not associated with age, gender diabetes, indication for surgery, material or type of bypass, number of preceding operations, or postoperative wound complications.