Research Paper Groundwater Quality Assessment Using Water Quality Index
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Central-Karachi
Central-Karachi 475 476 477 478 479 480 Travelling Stationary Inclass Co- Library Allowance (School Sub Total Furniture S.No District Teshil Union Council School ID School Name Level Gender Material and Curricular Sport Total Budget Laboratory (School Specific (80% Other) 20% supplies Activities Specific Budget) 1 Central Karachi New Karachi Town 1-Kalyana 408130186 GBELS - Elementary Elementary Boys 20,253 4,051 16,202 4,051 4,051 16,202 64,808 16,202 81,010 2 Central Karachi New Karachi Town 4-Ghodhra 408130163 GBLSS - 11-G NEW KARACHI Middle Boys 24,147 4,829 19,318 4,829 4,829 19,318 77,271 19,318 96,589 3 Central Karachi New Karachi Town 4-Ghodhra 408130167 GBLSS - MEHDI Middle Boys 11,758 2,352 9,406 2,352 2,352 9,406 37,625 9,406 47,031 4 Central Karachi New Karachi Town 4-Ghodhra 408130176 GBELS - MATHODIST Elementary Boys 20,492 4,098 12,295 8,197 4,098 16,394 65,576 16,394 81,970 5 Central Karachi New Karachi Town 6-Hakim Ahsan 408130205 GBELS - PIXY DALE 2 Registred as a Seconda Elementary Girls 61,338 12,268 49,070 12,268 12,268 49,070 196,281 49,070 245,351 6 Central Karachi New Karachi Town 9-Khameeso Goth 408130174 GBLSS - KHAMISO GOTH Middle Mixed 6,962 1,392 5,569 1,392 1,392 5,569 22,278 5,569 27,847 7 Central Karachi New Karachi Town 10-Mustafa Colony 408130160 GBLSS - FARZANA Middle Boys 11,678 2,336 9,342 2,336 2,336 9,342 37,369 9,342 46,711 8 Central Karachi New Karachi Town 10-Mustafa Colony 408130166 GBLSS - 5/J Middle Boys 28,064 5,613 16,838 11,226 5,613 22,451 89,804 22,451 112,256 9 Central Karachi New Karachi -

Sindh Government Hospital Liaquatabad, Karachi Basis
SINDH GOVERNMENT HOSPITAL LIAQUATABAD KARACHI. TERMS AND CONDITIONS OF FOR THE YEAR 2015-16 PURCHASE OF MEDICINES & LABORATORY KITS/CEHEMICALS 15 % / MEDICAL GASES/ DIET / MISCELLANEOUS ARTICLES REPAIR OF FURNITURE/TRANSPORT/ MACHINERY & EQUIPMENTS. SCHEDULE OF REQUIREMENTS & PRICES FOR THE CURRENT FINANCIAL YEAR 2015-16 TIME OF SALE FROM: Date of Publishing TIME OF OPENING TENDER: 31ST August, 2015 at 12:00 Noon Offer will remain open for 60 days from the date of opening. The tenders shall quote their prices inclusive of all duties/taxes/octori/transportation etc and all other expenses on free delivery to consignee’s end at Sindh Government Hospital Liaquatabad, Karachi basis. Price should be quoted in figures and words both, failing which the offer will be ignored. S# Nomenclature/Product Quantity Rate Amount Name Demanded Delivery Period: within 15 days Validity TILL 30 JUNE, 2016 1 The tender shall be submitted with all documents in sealed envelopes, with sealing wax. The envelope must contain tender enquiry number on the top.; the name of the manufacturer and the supplier should be affixed on the face of every envelope at the left side. 2 Tender must be filled in Blue or Black ink in the column provided/on separate letter head duly signed. 3 The tender must be free from erasing, cutting and over writing. In case of erasing, cutting and over writing authorized person should initial it. 4 The rates of each item should be written in figures as well as in words. Arithmetical errors will be rectified on there basis, if there is discrepancy between the unit price and the total price that is obtained by multiplying the unit price and the quantity, the unit price shall prevail and the total price shall corrected. -
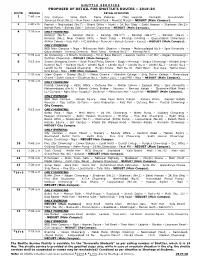
Shuttle Route
SHUTTLE SERVICES PROPOSED OF DETAIL FOR SHUTTLE’S ROUTES – 2019-20 ROUTE TIMINGS DETAIL OF ROUTES 1 7:40 a.m City Campus – Jama Cloth – Radio Pakistan – 7Day Hospital – Numaish – Gurumandir – Jamshed Road (No.3) – New Town – Askari Park – Mumtaz Manzil – NEDUET (Main Campus). 2 7:40 a.m Paposh – Nazimabad (No.7) – Board Office – Hydri – 2K Bus Stop – Sakhi Hassan – Shadman (No.2)– Namak Bank – Sohrab Goth – Gulshan Chowrangi – NEDUET (Main Campus). 4 7:20 a.m ONLY MORNING: Korangi (No.5) – Korangi (No.4) – Korangi (No.31/2) – Korangi (No.21/2) – Korangi (No.2) – Korangi (No.1, Near Chakra Goth) – Nasir Jump – Korangi Crossing – Qayyumabad Chowrangi – Akhtar Colony – Kala Pull – FTC Building – Nursery – Baloch Colony – Karsaz – NEDUET (Main Campus). ONLY EVENING: NED Main Campus – Nipa – Millennium Mall– Stadium – Karsaz – Mehmoodabad No.6 – Iqra University – Qayyumabad – Korangi Crossing – Nasir Jump – Korangi No.21/2 – Korangi No.5. 5 7:45 a.m 4K Chowrangi – 2 Minute Chowrangi – 5C-4 (Bara Market) – Saleem Centre – U.P Mor – Nagan Chowrangi – Gulshan Chowrangi – NEDUET (Main Campus). 6 7:15 a.m Shama Shopping Centre – Shah Faisal Police Station – Bagh-e-Korangi – Singer Chowrangi – Khaddi Stop – Korangi No.5 – Korangi No.6 – Landhi No.6 – Landhi No.5 – Landhi No.4 – Landhi No.3 – Landhi No.1 – Landhi No.89 – Dawood Chowrangi – Murghi Khana – Malir No.15 – Malir Hault – Star Gate – Natha Khan – Drig Road – Nipa – NED Main Campus. 7 7:35 a.m Islam Chowk – Orangi (No.5) – Metro Cinema – Abdullah College – Ship Owner College – Qalandarya Chowk – Sakhi Hassan – Shadman No.1 – Buffer Zone – Fazal Mill – Nipa – NEDUET (Main Campus). -

Appendix 4-1 Desirable Plan for Station Plaza Proposed in SAPROF-I Desirable Plaza Plans at Each Station Are Shown in the Following Figures with Satellite Images
APPENDIX 4-1 Desirable Plan for Station Plaza Proposed in SAPROF-I Preparatory Survey (II) on Karachi Circular Railway Revival Project Final Report Appendix 4-1 Desirable Plan for Station Plaza Proposed in SAPROF-I Desirable plaza plans at each station are shown in the following figures with satellite images. (1) Drigh Road Station Structure Station Plaza (2) Johar Source: SAPROF-I Figure 1 Desirable Station Plaza Plan (1/12) APP4-1-1 Preparatory Survey (II) on Karachi Circular Railway Revival Project Final Report (3) Alladin Park Station Structure Station Plaza (4) Nipa Source: SAPROF-I Figure 2 Desirable Station Plaza Plan (2/12) APP4-1-2 Preparatory Survey (II) on Karachi Circular Railway Revival Project Final Report (5) Gilani Station Structure Station Plaza (6) Yasinabad Source: SAPROF-I Figure 3 Desirable Station Plaza Plan (3/12) APP4-1-3 Preparatory Survey (II) on Karachi Circular Railway Revival Project Final Report (7) Liaquatabad Station Structure Station Plaza (8) North Nazimabad Source: SAPROF-I Figure 4 Desirable Station Plaza Plan (4/12) APP4-1-4 Preparatory Survey (II) on Karachi Circular Railway Revival Project Final Report (9) Orangi Station Structure Station Plaza (10) HBL Source: SAPROF-I Figure 5 Desirable Station Plaza Plan (5/12) APP4-1-5 Preparatory Survey (II) on Karachi Circular Railway Revival Project Final Report (11) Manghopir Station Structure Station Plaza (12) SITE Source: SAPROF-I Figure 6 Desirable Station Plaza Plan (6/12) APP4-1-6 Preparatory Survey (II) on Karachi Circular Railway Revival Project Final -

List of Branches Authorized for Overnight Clearing (Annexure - II) Branch Sr
List of Branches Authorized for Overnight Clearing (Annexure - II) Branch Sr. # Branch Name City Name Branch Address Code Show Room No. 1, Business & Finance Centre, Plot No. 7/3, Sheet No. S.R. 1, Serai 1 0001 Karachi Main Branch Karachi Quarters, I.I. Chundrigar Road, Karachi 2 0002 Jodia Bazar Karachi Karachi Jodia Bazar, Waqar Centre, Rambharti Street, Karachi 3 0003 Zaibunnisa Street Karachi Karachi Zaibunnisa Street, Near Singer Show Room, Karachi 4 0004 Saddar Karachi Karachi Near English Boot House, Main Zaib un Nisa Street, Saddar, Karachi 5 0005 S.I.T.E. Karachi Karachi Shop No. 48-50, SITE Area, Karachi 6 0006 Timber Market Karachi Karachi Timber Market, Siddique Wahab Road, Old Haji Camp, Karachi 7 0007 New Challi Karachi Karachi Rehmani Chamber, New Challi, Altaf Hussain Road, Karachi 8 0008 Plaza Quarters Karachi Karachi 1-Rehman Court, Greigh Street, Plaza Quarters, Karachi 9 0009 New Naham Road Karachi Karachi B.R. 641, New Naham Road, Karachi 10 0010 Pakistan Chowk Karachi Karachi Pakistan Chowk, Dr. Ziauddin Ahmed Road, Karachi 11 0011 Mithadar Karachi Karachi Sarafa Bazar, Mithadar, Karachi Shop No. G-3, Ground Floor, Plot No. RB-3/1-CIII-A-18, Shiveram Bhatia Building, 12 0013 Burns Road Karachi Karachi Opposite Fresco Chowk, Rambagh Quarters, Karachi 13 0014 Tariq Road Karachi Karachi 124-P, Block-2, P.E.C.H.S. Tariq Road, Karachi 14 0015 North Napier Road Karachi Karachi 34-C, Kassam Chamber's, North Napier Road, Karachi 15 0016 Eid Gah Karachi Karachi Eid Gah, Opp. Khaliq Dina Hall, M.A. -
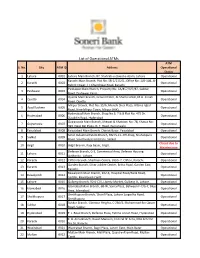
List of Operational Atms ATM S
List of Operational ATMs ATM S. No. City ATM ID Address Operational Status 1 Lahore 0001 Lahore Main Branch, 87, Shahrah-e-Quaid-e-Azam, Lahore. Operational Karachi Main Branch, Plot No: SR-2/11/2/1, Office No: 105-108, Al- 2 Karachi 0002 Operational Rahim Tower, I.I. Chundrigar Road, Karachi Peshawar Main Branch, Property No: CA/457/3/2/87, Saddar 3 Peshawar 0003 Operational Road, Peshawar Cantt. Quetta Main Branch, Ground Floor, Al-Shams Hotel, M.A. Jinnah 4 Quetta 0004 Operational Road, Quetta. Mirpur Branch, Plot No: 35/A, Munshi Sher Plaza, Allama Iqbal 5 Azad Kashmir 0005 Operational Road, New Mirpur Town, Mirpur (AJK) Hyderabad Main Branch, Shop No.6, 7 & 8 Plot No. 475 Dr. 6 Hyderabad 0006 Operational Ziauddin Road, Hyderabad Gujranwala Main Branch, Khewat & Khatooni No: 78, Khasra No: 7 Gujranwala 0007 Operational 393, Near Din Plaza, G. T. Road, Gujranwala 8 Faisalabad 0008 Faisalabad Main Branch, Chiniot Bazar, Faisalabad Operational Small Industrial Estate Branch, BIV-IS-11--RH-Shop, Shahabpura 9 Sialkot 0009 Operational Road, Small Industrial Estate, Sialkot Closed due to 10 Gilgit 0010 Gilgit Branch, Raja Bazar, Gilgit. Maintenance Defence Branch, G-3, Commercial Area, Defence Housing 11 Lahore 0011 Operational Authority, Lahore. 12 Karachi 0012 Clifton Branch, Shadman Centre, Block-7, Clifton, Karachi. Operational Garden Branch, Silver Jubilee Center, Britto Road, Garden East, 13 Karachi 0013 Operational Karachi. Rawalpindi Main Branch, 102-K, Hospital Road/Bank Road, 14 Rawalpindi 0014 Operational Saddar, Rawalpindi Cantt. 15 Lahore 0015 Gulberg Branch, 90-B-C/II, Liberty Market, Gulberg III, Lahore. -

List of Reimbursement Hospital
LIST OF AUTHORIZED GOVT. / PRIVATE HOSPITALS (ON REIMBURSEMENT SYSTEM) Sr. Address No. Name of Hospital Federal Govt. 1. Combined Military Hospital Malir Cantt, Karachi. 2. J.P.M.C. Rafique Shaheed Road, Karachi. 3. P.N.S. Shifa. Defence Society, Karachi. 4. Shoukat Omer Memorial Hospital Shah Faisal Colony, Karachi. Sindh Govt. 5. Civil Hospital Baba-e-Urdu Road, Karachi. 6. Kulsum Bai Valika Hospital Mangopir Rd. S.I.T.E. , Karachi. 7. Nazimabad Chest Clinic Nazimabad, Karachi. 8. Ojah Institute of Chest Diseases Univesity Road, Mosamiat, Karachi. 9. Sindh Govt. Hospital Korangi. Korangi, Karachi. 10. Sindh Govt. Hospital Liaquatabad Liaquatabad, Karachi. 11. Sindh Govt. Hospital Lyari Lyari, Karachi. 12. Sindh Govt. Hospital New Karachi New Karachi. 13. Sindh Govt. Qatar Hospital Orangi Town, Karachi. 14. Sindh Services Hospital M. A Jinnah Road, Karachi. 15. Skin & Social Hygiene Centre Preedy Street, Saddar, Karachi. 16. Social Security Hospital Landhi, Karachi. 17. National Institute of Cardio vascular Raffique Shaheed Rd, Bizerta Lines, Cantt, Karachi Diseases (NICVD) Local Govt./ City Govt. 18. Abbasi Shaheed Hospital Nazimabad No.5, Karachi. 19. Spencer Eye Hospital Lee Market, Karachi. Karachi Institute of Heart Diseases ST-15, Block-16, Near UBL Sports Complex, 20. Federal (KIHD) B. Area, Karachi. Dental Surgery. 21. Rehman Dental Surgery Maskan Chowrangi Gulshan-e Iqbal, Karachi. Private 22. A.O Clinic Nazimabad No.7, Karachi. 23. Aga Khan Maternity Home Karimabad, F. B Area, Karachi. Karimabad 24. Aga Khan Maternity Home Kharadar Kharadar, Karachi. 25. Akhtar Eye Hospital Gulshan-e Iqbal, Karachi. 26. Al-Ain Eye Hospital P.E.C.H.S. -

S.No Folio No. Name of Shareholder Address Nature of Amount Amount
sanofi-aventis Pakistan Limited Details of Unclaimed Dividend Amount of Nature of Unclaimed S.No Folio No. Name of Shareholder Address Amount Dividend (Rupees) 1 10 MR. RAFIQUE 223/B, KCH SOCIETY ADAMJEE NAGAR KARACHI-8 Dividend 35,172 2 28 MR. MOHAMMED MATIUR RAHMAN B-21, AL-AZAM SQUARE, FEDERAL B AREA, KARACHI-19 Dividend 32,365 3 59 MR. RAZZAK AHMED BAVA 106-B, MADINA TAA PLOT NO. 538/13, GUL MOHAR STREET GARDEN EAST KARACHI-74800 Dividend 2,963 4 112 MR. MOHD ABID MAJEED SOLEJA, HILL AVENUE 167 JUSTICE INAMULLAH ROAD OFF SHAHEED-E-MILLAT ROAD BLOCK 7 & 8 KARACHI-74800 Dividend 22,111 5 170 MR. HAROON LATIF 31, HAROON BROS BUILDING, M.A. JINNAH ROAD, KARACHI. Dividend 35,563 6 276 MR. MEHDI MOHSIN, 29 F.C.C. GULBERG, LAHORE. Dividend 5,154 7 280 MR. Z. AHMED, 156-B BLOCK H, NORTH NAZIMABAD, KARACHI. Dividend 26,042 8 281 MS. SHAHNAZ DAWOOD 73 P.M.A. BUILDING AWAN-E-TIJARAT ROAD KARACHI. Dividend 32,579 9 284 MRS. JENABAI ALIBHAI, B-175, BLOCK C, NORTH NAZIMABAD, KARACHI. Dividend 30,424 10 286 MR. VILAYAT A-10, AL-REHMAT GARDEN WEST, KARACHI. Dividend 27,480 11 287 MS. ISMET, 42, AL-AMIN FLATS, ALFRED STREET, KARACHI. Dividend 27,480 12 289 MR. ZULFIQAR, CUMBER STUDIO, 171/1, BRITTO ROAD, KARACHI. Dividend 13,482 13 290 MR. ARSHAD AKBAR ALI, B-175, BLOCK C, NORTH NAZIMABAD, KARACHI.33 Dividend 30,224 14 334 MRS. NAZIRUDDIN, C/81, BLOCK-9, GULSHAN-E-IQBAL KARACHI-75300. Dividend 7,452 15 360 MR. -

1. Persons Interviewed
Karachi Transportation Improvement Project Final Report APPENDIX-1 MEETINGS 1. Persons Interviewed During the Phase 1 study period, which is Karachi Transport Master Plan-2030, JICA Study Team (JST) has been visited different organizations and Departments to collect data and also meet the officials. The list of these officials and their department/organization are given below 1.1 CDGK Administrator/ DCO, City District Government Karachi 1.1.1 KMTC Director General, Karachi Mass Transit Cell, CDGK Director, (Planning & Coordination) Karachi Mass Transit Cell, CDGK. Director (T), KMTC, CDGK 1.1.2 Master Plan Executive District Officer, Master Plan Group of Offices, CDGK District Officer, Master Plan Group of Offices, CDGK 1.1.3 Transport & Communication Executive District Officer, Transport Department , CDGK District Officer (Parking & Terminal Management), Transport & Communication Department (TCD), CDGK District Officer, Policy, Planning & Design, Transport & Communication Dept. CDGK 1.1.4 Education Department Executive District Officer, Education(School) , CDGK 1.1.5 Works & Service Department Executive District Officer, W&S , CDGK 1.2 DRTA Superintendant, District Regional Authority, CDGK 1.3 Town Administration Administrator, Keamari Town Administrator, Baldia Town Administrator, Bin Qasim Town Administrator, Gulberg Town Administrator, Gadap Town Administrator, Gulshan Town Administrator, Jamshed Town Administrator, Korangi Town Administrator, Landhi Town Administrator, Liaquatabad Town Appendix 1 - 1 Karachi -

Studies in Planning and Management Transition in the Karachi Metropolitan Region
COMMENTARY From development authorities to democratic institutions: Studies in planning and management transition in the Karachi Metropolitan Region Commonwealth Journal of Local Governance Issue 7: November 2010 http://epress.lib.uts.edu.au/ojs/index.php/cjlg Noman Ahmed Department of Architecture and Planning NED University of Engineering and Technology Karachi, Pakistan 1. Introduction Karachi is a relatively young metropolis. The city is about three centuries old, founded as a port by local Hindu merchants and traders. The British conquered the city in 1839, occupied the entire principality of Sindh in 1843 and designated Karachi as headquarters of the territory. The city experienced different phases of growth. When Pakistan came into being in 1947, a large number of refugees entered the city and the population jumped from 435,000 in 1947 to 1,050,000 in 1951. Karachi has remained the primate city of Pakistan, with a high annual growth rate of 5-7 percent per annum (Ahmed 1998). Current estimates suggest that the city has about 17 million inhabitants. Karachi houses 7 per cent of the total population and 23 per cent of the urban population of Pakistan. Its current rate of growth is estimated at around 5 per cent, of which 3 per cent is due to natural increase and 2 per cent to migration from the other parts of the country. Karachi provides 25 per cent of federal revenue and 15 per cent of Pakistan’s gross domestic product (GDP). In addition, 50 per cent of the country’s bank deposits and 72 per cent of all issued capital is contributed by the city (Hasan 2010; CDGK 2008). -

Karachi Heatwave Management Plan: a Guide to Planning and Response
Karachi Heatwave Management Plan: A Guide to Planning and Response Commissioner Karachi Purpose of the Document This document, Karachi Heatwave Management Plan, outlines what should happen before, during and after periods of extreme heat in Karachi. It sets out strategies that government and non-government agencies will adopt to prevent heat-related illnesses and deaths in Karachi and capacitate the public, particularly the most vulnerable residents, to take protective action. The Plan describes actions of implementation partners to ensure (1) information on weather conditions and heat health is timely and specific, (2) organizations have the capacity to respond according to their roles, and (3) strategies and actions enabling increase in effectiveness over time. In June 2015 Karachi City experienced a severe heatwave that caused over 1,200 deaths and over 50,000 cases of heat illness. The heatwave caught all levels of government and first responders off-guard, highlighting the need for inter-agency coordination, clarity in roles, and a well-publicized trigger to activate a planned response. To address this need and to prevent health impacts from future heatwaves as climate change intensifies, the Commissioner Office Karachi requested support from the Climate and Development Knowledge Network (CDKN) to develop a heatwave management plan. Karachi’s first Heatwave Management Plan is the result of a technical assistance project delivered by national and international experts between October 2016 and May 2017, working closely with the Commissioner Office and other stakeholders. The Plan will be subject to an annual performance review and updated versions will be available to implementation partners accordingly. ii | P a g e Table of Contents List of Figures ...................................................................................................................... -
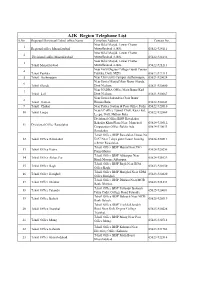
Copy of Compiled List Phone Nos BISP ALL Regions Dated 21.05
AJK Region Telephone List S.No Regioanl/Divisioanl/Tehsil office Name Complete Address Contact No. Near Bilal Masjid, Lower Chatter 1 Regional office Muzaffarabad Muzaffarabad AJ&K. 05822-924111 Near Bilal Masjid, Lower Chatter 2 Divisional office Muzaffarabad Muzaffarabad AJ&K. 05822-924132 Near Bilal Masjid, Lower Chatter 3 Tehsil Muzaffarabad Muzaffarabad AJ&K. 05822-921213 Near Girls Degree College Gandi Peeran 4 Tehsil Patikka Patikka, Distt. MZD. 05822-922113 5 Tehsil Authmaqam Near University Campus Authamaqam, 05821-920024 Near Jamia Masjid Main Bazar Sharda, 6 Tehsil Sharda Distt Neelum. 05821-920800 Near NADRA Office Main Bazar Kail 7 Tehsil kail Distt Neelum. 05821-920667 Near Jamia Sakandria Chok Bazar 8 Tehsil Hattian Hattian Bala. 05822-922643 9 Tehsil Chakar Near Police Station & Post Office Pothi 05822-922010 NearAC office Tunnel Chok, Kaser kot, 10 Tehsil Leepa 05822-922869 Leepa, Distt. Hattian Bala. Divisional Office BISP Rawalakot Bahadar Khan Plaza Near Muncipal 05824-920512, 11 Divisional Office Rawalakot Corporation Office Baldia Ada 05824-920033 Rawalakot. Tehsil Office BISP Rawalakot House No 12 Tehsil Office Rawalakot D-97 Near 7 days guest house housing 05824-920511 scheme Rawalakot. Tehsil Office BISP Hajira Near PSO 13 Tehsil Office Hajira 05824-920256 Pump Hajira. Tehsil Office BISP Abbaspur Near 14 Tehsil Office Abbas Pur 05824-921029 Hanfi Mosque Abbaspur. Tehsil Office BISP Bagh Near BDA 15 Tehsil Office Bagh 05823-920150 Office Bagh. Tehsil Office BISP Harighel Near SDM 16 Tehsil Office Harighel 05823-920820 Office Harighel. Tehsil Office BISP Dhirkot Near MCB 17 Tehsil Office Dhirkot 05823-921233 Bank Dhirkot. Tehsil Office BISP Pallandri Balouch 18 Tehsil Office Palandri 05825-920081 Palza Cadet College Road Palandri.