Publications Citing Vapourtec
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

University at Buffalo, Chemical and Biological Engineering February 2, 2021
University at Buffalo, Chemical and Biological Engineering February 2, 2021 CURRICULUM VITAE Gang Wu, Ph. D., Professor Department of Chemical and Biological Engineering University at Buffalo (UB), The State University of New York (SUNY), Buffalo, New York, USA E-mail: [email protected]; Phone: 716-645-8618 (office) ; 803-338-4924 (cell) Web: www.cbe.buffalo.edu/wu Education • 2004. Ph.D.: Environmental Engineering, Harbin Institute of Technology, Harbin, China. • 1999. M.S.: Applied Chemistry, Harbin Institute of Technology, Harbin, China. • 1997. B.S: Electrochemical Engineering, Harbin Institute of Technology, Harbin, China. Employment History • Aug 2020- present, Professor, University at Buffalo, SUNY, USA • Aug 2018-Aug 2020, Associate Professor, University at Buffalo, SUNY, USA • Aug 2014-Aug 2018, Assistant Professor, University at Buffalo, SUNY, USA • May 2010-Aug 2014, Staff Scientist, Los Alamos National Laboratory (LANL), USA • Jan 2008-May 2010, Postdoc, Los Alamos National Laboratory, USA • Feb 2006-Jan 2008, Postdoc, University of South Carolina, USA • Jan 2004-Jan 2006, Postdoc, Tsinghua University, Beijing, China Major Research Interest • Electrochemical Science and Engineering for Energy Technologies; • Electrocatalysis and photocatalysis for clean energy conversion: fuel cells, electrolyzers, CO2 reduction; electrosynthesis; • Electrochemical energy power sources for energy storage: batteries and supercapacitors; • Renewable fuel: NH3 (electrosynthesis, oxidation, and carking for H2 generation). Key Achievements and Recognition • Dr. Wu is internationally recognized as the leading researcher in the field of fuel cells and other sustainable electrochemical energy technologies. • Awarded more than $5.0 M in grant funding from federal agencies (DOE and NSF) since joining UB in August 2014. Those 14 projects (3 NSF and 11 DOE) focus on the development of advanced materials for electrochemical energy conversion and storage technologies such as fuel cells, water splitting, batteries, and renewable fuel (e.g., NH3). -

Socit Chimique De France 2014 Prize Winners
Angewandte. Angewandte News Chemie Socit Chimique de France 2014 Prize Nazario Martn (Universidad Complutense de Winners Madrid) is the winner of the Prix franco-espagnol Awarded … Miguel Cataln–Paul Sabatier. Martn was featured The Socit Chimique de France has announced its here when he was awarded the 2012 EuCheMS 2014 prize winners. We congratulate all the awar- Lectureship.[4a] Martn is on the International dees and feature our authors and referees here. Advisory Boards of Chemistry—An Asian Journal, Max Malacria (Institut de Chimie des Substan- ChemPlusChem, and ChemSusChem. His report on ces Naturelles; ICSN) is the winner of the Prix modified single-wall nanotubes was recently fea- Joseph Achille Le Bel, which is awarded to tured on the cover of Chemistry—A European recognize internationally recognized research. Mal- Journal.[4b] acria studied at the Universit Aix-Marseille III, Michael Holzinger (Universit Joseph Four- where he completed his PhD under the supervision nier, Grenoble 1; UJF) is the winner of the Prix M. Malacria of Marcel Bertrand in 1974. From 1974–1981, he jeune chercheur from the Analytical Chemistry was matre-assistant with Jacques Gore at the Division. Holzinger carried out his PhD at the Universit Claude Bernard Lyon 1 (UCBL), and Friedrich-Alexander-Universitt Erlangen-Nrn- from 1981–1983, he carried out postdoctoral berg. After postdoctoral research at the Universit research with K. Peter C. Vollhardt at the Univer- Montpellier 2 (UM2) and the Max Planck Institute sity of California, Berkeley. He returned to the for Solid-State Research, and working at Robert UCBL as matre de conferences in 1983, and was Bosch, he joined Serge Cosniers group at the UJF made professor at the Universit Pierre et Marie as a CNRS charg de recherche. -
2019 Wiley Title List
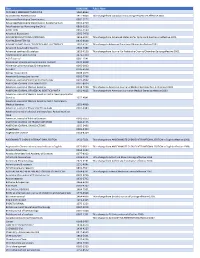
Title ISSN/ISBN Public Note ACADEMIC EMERGENCY MEDICINE 1069-6563 ACCOUNTING PERSPECTIVES 1911-382X Title change from Canadian Accounting Perspectives effective 2007. Acta anaesthesiologica Scandinavica 0001-5172 Acta anaesthesiologica Scandinavica. Supplementum 0515-2720 Acta Paediatrica: Nurturing the Child 0803-5253 ADDICTION 0965-2140 Advanced Biosystems 2366-7478 ADVANCED FUNCTIONAL MATERIALS 1616-301X Title change from Advanced Materials for Optics and Electronics effective 2001. ADVANCED MATERIALS 0935-9648 ADVANCED MATERIALS FOR OPTICS AND ELECTRONICS 1057-9257 Title change to Advanced Functional Materials effective 2001. Advanced Sustainable Systems 2366-7486 Advanced synthesis & catalysis 1615-4150 Title change from Journal für Praktische Chemie/Chemiker-Zeitung effective 2001. AEM Education and Training 2472-5390 AIChE Journal 0001-1541 Alcoholism: clinical and experimental research 0145-6008 Alimentary pharmacology & therapeutics 0269-2813 ALLERGY 0105-4538 Allergy. Supplement 0108-1675 American Business Law Journal 0002-7766 American Journal of Community Psychology 0091-0562 AMERICAN JOURNAL OF HEMATOLOGY 0361-8609 American Journal of Medical Genetics 0148-7299 Title change to American Journal of Medical Genetics Part A effeective 2003. AMERICAN JOURNAL OF MEDICAL GENETICS PART A 1552-4825 Title change from American Journal of Medical Genetics effective 2003. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 1552-4841 American Journal of Medical Genetics Part C: Seminars in Medical Genetics 1552-4868 American Journal of Physical Anthropology 0002-9483 American journal of physical anthropology. Annual meeting issue American Journal of Political Science 0092-5853 AMERICAN JOURNAL OF TRANSPLANTATION 1600-6135 AMERICAN JOURNAL ON ADDICTIONS 1055-0496 Anaesthesia 0003-2409 Angewandte Chemie 0044-8249 ANGEWANDTE CHEMIE INTERNATIONAL EDITION 1433-7851 Title change from ANGEWANDTE CHEMIE INTERNATIONAL EDITION in English effective 1998. -

Curriculum Vitae for Narayan S. Hosmane
CURRICULUM VITAE NARAYAN S. HOSMANE HOME ADDRESS: BUSINESS ADDRESS: 663 Teal Court Dept. of Chemistry & Biochemistry DeKalb, Illinois 60115-6201 Northern Illinois University Phone: (815) 754-1205 DeKalb, Illinois 60115-2862 E-Mail: [email protected] Tel: 815-753-3556 / Fax: 815-753-4802 PLACE OF BIRTH: CITIZENSHIP: Gokarn, Southern India U.S.A. EDUCATION B.S. 1968 Karnatak University, Dharwar, India Organic, Inorganic and Physical Chemistry M.S. 1970 Karnatak University, Dharwar, India Inorganic Chemistry Ph.D. 1974 University of Edinburgh, Edinburgh, Scotland Organometallic/Inorganic Chemistry Ph.D. Thesis: Some Reactions of Stannic Chloride with Silicon Hydrides and Some Novel Group IV Derivatives of Mercury. E.A.V. Ebsworth as Research Advisor. RECORD OF EMPLOYMENT Postdoctoral Research Assistant, Queen’s University of Belfast 1974-75. Full-time research under the supervision of Professor Frank Glockling in the areas of methylmercurials in relation to toxicology and environmental pollution. Research Scientist, Catalysis Section, Lambeg Industrial Research Institute, Northern Ireland 1975-76. Supervised a group of chemists researching on odor and pollution control. Research Associate, Auburn University, Auburn, Alabama 1976-77. Full-time research under the supervision of Professors Frederic A. Johnson and William E. Hill in the area of synthetic and -2 kinetic studies of B10H10 and C2B10-carboranes. Research Associate, University of Virginia, Charlottesville, Virginia, 1977-79. Full-time research under the supervision of Professor Russell N. Grimes in the area of carboranes and metallacarboranes. Assistant Professor, Virginia Polytechnic and State University, Blacksburg, Virginia, 1979-82. Assistant Professor (tenure track), Southern Methodist University, 1982-1986. Sabbatical Leave from SMU, Sept. -

Univ.-Prof. Cristian A. STRASSERT – List of Publications
Univ.-Prof. Cristian A. STRASSERT – List of publications Peer-reviewed publications 102. Encapsulation of Phosphorescent Pt(II) Complexes in Zn-Based Metal–Organic Frameworks toward Oxygen-Sensing Porous Materials. Knedel, T. O.; Buss, S.; Maisuls, I.; Daniliuc, C. G.; Schlüsener, C.; Brandt, P.; Weingart, O.; Vollrath, A.; Janiak, C.; Strassert, C. A. Inorganic Chemistry 2020, accepted, doi.org/10.1021/acs.inorgchem.0c00678 101. Cyclometalated Pt Complexes of CNC Pincer Ligands: Luminescence and Cytotoxic Evaluation. Garbe, S.; Krause, M.; Klimpel, A.; Neundorf, I.; Lippmann, P.; Ott, I.; Brünink, D.; Strassert, C. A.; Doltsinis, N. L.; Klein, A. Organometallics 2020, 39, 746 100. Synthesis of Small-Molecule Fluorescent Probes for the In Vitro Imaging of Calcium- Activated Potassium Channel KCa3.1. Brömmel, K.; Maskri, S.; Maisuls, I.; Konken, C. P.; Rieke, M.; Pethö, Z.; Strassert, C. A.; Koch, O., Schwab, A.; Wünsch, B. Angewandte Chemie International Edition 2020, 59, 8277 - Synthesis of Small-Molecule Fluorescent Probes for the In Vitro Imaging of Calcium-Activated Potassium Channel KCa3.1. Brömmel, K.; Maskri, S.; Maisuls, I.; Konken, C. P.; Rieke, M.; Pethö, Z.; Strassert, C. A.; Koch, O., Schwab, A.; Wünsch, B. Angewandte Chemie 2020, 132, 8354 99. Understanding the Role of Chalcogens in Ether-Based Luminophores with Aggregation- Induced Fluorescence and Phosphorescence. Riebe, S.; Wölper, C.; Balszuweit, J.; Hayduk, M.; Gutierrez Suburu, M.; Strassert, C. A.; Doltsinis, N. L.; Voskuhl, J. ChemPhotoChem 2020, 4, 1 98. Gadolinium Photocatalysis: Dearomative [2+2] Cycloaddition/Ring-Expansion Sequence with Indoles. Ma, J.; F. Schäfers, F.; Daniliuc, C. G.; Bergander, K.; Strassert, C. A.; Glorius, F. -

General Information on Accepted Manuscripts Dear Author
General information on accepted manuscripts Dear author, Thank you for selecting ChemSusChem for your next publication. Now that your manuscript has been accepted, we kindly ask you to carefully follow the instructions provided in this document. This will enable faster processing and thereby minimize publication times. This document contains the following information: 1 Checklist: Uploading production material ............................................................................................. 2 2 Preparing the text .................................................................................................................................. 3 3 Preparing the graphics .......................................................................................................................... 8 4 Submitting Cover Artwork ................................................................................................................... 16 Visit our homepage or contact the Editorial Office if you have any additional questions: Phone (Kirsten Gumbel) (+49) 6201-606-659 E-mail: [email protected] Homepage: http://www.chemsuschem.org We look forward to the publication of your manuscript! With kind regards, David Smith Editor-in-Chief Halgard Staesche Managing Editor Lauren Scholz Associate Editor Kirsten Gumbel Editorial Assistant Melanie Friese Production Manager ChemSusChem is a journal from the portfolio of the 16 national chemical societies that together form ChemPubSoc Europe. The journal is published by Wiley-VCH Verlag -

Stahl Journal Abbreviations Masterlist
Please use a standard format for naming pdf files: YYJJ [Author's last name] [full or abbreviated title of article] YY = year of journal article; JJ = 2-4 letter abbreviation of journal name Full Journal Title Journal Abbrev. Stahl Abbrev. Accounts of Chemical Research Acc. Chem. Res. ACR ACS Applied Materials & Interfaces ACS Appl. Mater. Interfaces AMI ACS Catalysis ACS Catal. CAT ACS Central Science ACS Cent. Sci. CTS ACS Combinatorial Science ACS Comb. Chem. CBC ACS Energy Letters ACS Energy Lett. EL ACS Sustainable Chemistry & Engineering ACS Sustainable Chem. Eng. SCE ACS Omega ACS Omega AO ACS Medicinal Chemistry Letters ACS Med. Chem. Lett. MCL Acta Chemica Scandinavica Acta Chem. Scand. ACS Acta Crystallographica Section A: Foundations Acta Crystallogr., Sect. A: Found Chrstallogr. ACGA Acta Crystallographica Section B: Structural Science Acta Crystallogr., Sec. B: Struct. Sci ACGB Acta Crystallographica Section C: Chrstal Structure Communications Acta Crystallogr., Sec. C: Cryst. Struct. Commun. ACGC Acta Crystallographica Section D: Biological Crystallography Acta Crystallogr., Sec. D:Biol. Crystallogr. ACGD Acta Crystallographica Section E: Structure Reports Online Acta Crystallogr., Sec. E: Struct. Rep. Online ACGE Advanced Energy Materials Adv. Energy Mater. AEM Advanced Materials Adv. Mater AM Advanced Synthesis and Catalysis Adv. Synth. Catal. ASC Advances in Inorganic Chemistry Adv. Inorg. Chem AIC Analytical Chemistry Anal. Chem. ANC Angewandte Chemie Angew. Chem. AC Angewandte Chemie International Edition Angew. Chem., Int. Ed. ACIE Applied Catalysis A: General Appl. Catal., A ACA Applied Catalysis B: Environmental Appl. Catal.,B ACB Applied Energy Appl. Energy AE Australian Journal of Chemistry Aust. J. Chem. AJC Asian Journal of Organic Chemistry Asian. -

Gesamtzeitschriftenverzeichnis Der Fachbibliothek Chemie Und Pharmazie
Gesamtzeitschriftenverzeichnis der Fachbibliothek Chemie und Pharmazie *Mit [Magazin] bzw. [Altbestandsmagazin] gekennzeichnete Bestände sind in nicht frei zugängliche Magazine ausgelagert. Bitte wenden Sie sich zwecks Benutzung solcher Bestände an die Theke. Abridged scientific publications from 9.1925 - 12.1928 VN 5850 E13 the Kodak Research Laboratories Abstracts of declassified documents 1.1947(1955),Jul. - 2.1948(1955) UA 1124.5 [Magazin]* Academie des Sciences <Paris>: 274.1972 - 281.1975 TA 1030.C Comptes rendus des séances de l'Academie des Sciences / C Academie des Sciences <Paris>: 86.1878 - 194.1932; 196.1933 - TA 1030 Comptes rendus hebdomadaires des 212.1941; 214.1942 - 216.1943 séances de l'Academie des Sciences Kommentar: 152.1911, April - Juni vermisst Accounts of chemical research 1.1968 - 42.2009 VA 1370 Acta chemica Scandinavica 1.1947 - 27.1973 VA 1400 Acta chemica Scandinavica / A 28.1974 - 42.1988 VA 1400.A Acta chemica Scandinavica / B 28.1974 - 42.1988 VA 1400.B Acta chemica Scandinavica 43.1989 - 53.1999 VA 1401 Acta crystallographica 1.1948 - 23.1967 VA 1455 Acta crystallographica / A 24.1968 - 52.1996 VA 1455.A Acta crystallographica / B 24.1968 - 52.1996 VA 1455.B Acta crystallographica / C 39.1983 - 61.2005 VA 1455.C Acta crystallographica / D 49.1993 - 52.1996 VA 1455.D Acta pharmaceutica Nordica 1.1989 - 4.1992 VA 1471 Acta pharmaceutica Suecica 1.1964 - 25.1988 VA 1470 Acta pharmaceutica technologica [6.]1960 - 88.2014 VA 1480 Acta pharmaceutica technologica / 1.1976 - 8.1979 VA 1480.0 Supplement Acta physicochimica URSS 1.1934 - 13.1940 VA 1495 Actinides reviews 1.1967/68(1967/71) VA 1507 Advanced engineering materials 1.1999 - 8.2006 VA 1507.4 Advanced materials 1988,Mai-Dez.; 1.1989 - 21.2009 VA 1507.5 Kommentar: 1988,Mai-Dez.; 1.1989 mit entspr. -

18-19 Chemistry Prog Review Part 2
Appendix I Chemistry FTES, FTEF, and FTES:FTEF 45 46 Appendix*: Chemistry FTES, FTEF, and FTES:FTEF Physical and Environmental Sciences Departmental Data, 2018 Ratiooffull-timeequ1valentstudents(FTES)tofull-timeequ1valentfaculty(FTEF) - - ·-·--- ·· - - - -- - - -- --· - - - - - -- - - -·- 2013-14 2014-15 2015-16 2016-17 2017-18 FTES FTE F FTES:ITTF FTES FTEF ms:FTEF FTES FTEF FTES:FTEF FTES FTEF FTES:FTEF FTES FTEF FTES:FTEF I CHEM 195.1 7.3 26.8 195.S 7.4 26.'1 197.7 7.8 25 .S 211.2 7.8 27.1 '. 227 .6 . 8.3 27.6 48 Appendix II Course Evaluation Form 49 50 •• • • • - Colorado Mesa University Faculty Evaluation CRN Western Colorado Community College Faculty Evaluation tnstructor Namo: ------ Course· Section {i; c11a ,i,-i- -\;·:-<.u1 ). - NOTE TO STUDENTS· Your responses a.re .inonymm,s TI1e results will no1 be returned to tne professor - unlll AFTER 91ades have been pcs:ed. IMPORTANT! Th,s clocuniem wdf !Je scanned for d,ll,, 01i\ry. P-leas~ eomptatf"Y Iii' n lhe circle of your selO?cllon with pencil or a black or blue pen - OPTtONAl DATA SECTION: Your responses to the following items are optloNJI. 1. Gender 2. Classificallon J . Type of Course 4. Degree - Male Fieshman Genern• Education Certifk:ate BS - , Femctle Sopr,omme Heau,recr f..:i: 1.1a101 AA 8$N , T- Junior · !: Flec:,·,e m Maier AS MA Sernor ·,-. F.leGhf' Non ll"aJor Al,S \~l:!A - Unclassified BA t,'.SN Graduate BAS ONP BBA Undeclared - BFA N/A - 5. Department of Major (i, E.Kpec;ted grade .··M Mui.·c for this course: ,. -

Zeitschriftenliste / Fachbereich Chemie, Bibliothek
Zeitschriftenliste / Fachbereich Chemie, Bibliothek Titel Bestand Bemerkungen Regal Standort Abhandlungen aus dem Gebiete der Naturwissenschaften 10 (1887); 21(1927),3-4; (1929),3-4 51 Magazin Abhandlungen und Verhandlungen des Naturwissenschaftlichen N.F. 2.1957(1958) - 4 (1959); 6 (1961) - 18/19 Vorg.: Abhandlungen aus dem Gebiete der 51 Magazin Vereins in Hamburg (1973/74) Naturwissenschaften Vorg. u. Forts.: Verhandlungen des Naturwissenschaftlichen Vereins in Hamburg Abhandlungen und Verhandlungen des Naturwissenschaftlichen 3 (1960) - 5 (1961); 15 (1972) 51 Magazin Vereins in Hamburg. Neue Folge. / Supplement Accounts of chemical research 1 (1968) - 45 (2012) 137 LS 1 Acta biochimica polonica 10 (1963) - 41 (1994) 130 LS 1 Acta chemica Scandinavica 1 (1947) - 27(1973); 43 (1989) - 53 (1999) Forts: Acta chemica Scandinavica / A + B 36 Magazin Acta chemica Scandinavica / A + B 28 (1974) - 36 (1982) Vorg. u. Forts.: Acta chemica Scandinavica 36 Magazin Acta crystallographica 1 (1948) - 23 (1967) Forts.: Acta crystallographica / A 46 WWLS Forts.: Acta crystallographica / B Acta crystallographica / A 24 (1968) - 39 (1983) Vorg.: Acta crystallographica 46 WWLS Acta crystallographica / B 24 (1968) - 39 (1983) Vorg.: Acta crystallographica 46 WWLS Acta pharmaceutica technologica 21 (1975) - 36 (1990) Vorg.: Informationsdienst APV 38 WWLS Forts.: European journal of pharmceutics and biopharmaceutics Acta polymerica 30 (1979) - 35 (1984) Vorg.: Faserforschung und Textiltechnik 143 LS 1 Advanced engineering materials 1 (1999),2; 2 (2000),1-2u.4-6, 8-12; 3 (2001),1-6, 8- 135 LS 1 12 - 6 (2004),9 Advanced functional materials 11 (2001),1-5 ; 14 (2004) - 19 (2009) 24 WWLS Advanced materials 1988, Mai - Dez; 2 (1990) - 21 (2009) Beil. -

New Members of the Editorial Board and International Advisory Board of Angewandte Chemie
Angewandte. Angewandte News Chemie New Members of the Editorial Board BioChem and is on the International Advisory and International Advisory Board Board of the Israel Journal of Chemistry. Stefan Grimme (University of Bonn) studied at Editorial Board the Technische Universitt Braunschweig, where he was awarded his PhD in 1991 for work The Editorial Board advises the editorial team on supervised by Herbert Dreeskamp. He completed all important issues and are selected by the Board his habilitation 1997 in the group of Sigrid Peyerim- of the Gesellschaft Deutscher Chemiker (GDCh; off at the University of Bonn, and in 2000, he was Featured … German Chemical Society) upon recommendation made Chair of Theoretical Organic Chemistry at by the Editorial Board and the editorial team. the University of Mnster. He moved to the Chair After 19 years on the Editorial Board (and 10 years of Theoretical Chemistry at the University of Bonn as its Chairman) FranÅois Diederich will now join in 2011. Grimmes research involves quantum- the International Advisory Board (see below). chemical methods, density functional theory and Hartmut Michel, Walter Thiel, and Otto Wolfbeis electronic structure, and theoretical electronic have completed two terms and will now leave the spectroscopy and thermochemistry.[4] Grimme is Editorial Board, and we thank them all for their also on the Editorial Advisory Board of Chemis- commitment to the journal. Matthias Beller, Stefan tryOpen. Buchholz, Claus Feldmann, Martin Suhm, and Hansjçrg Grtzmacher (ETH Zurich) studied F. Diederich Herbert Waldmann have all been elected to at the University of Gçttingen, where he completed another term. Alois Frstner (Max Planck Institute his PhD (supervised by Herbert W. -

French Chemical Society 2018 Prix Franco-Chinois
Angewandte News Chemie SociØtØ Chimique de France Prizes 2018 Chemistry—A European Journal on the synthesis of polyfunctionalized triaryllanthanum reagents,[3a] Awarded … TheSociØtØ Chimique de France (French Chemical and in Angewandte Chemie on cobalt-catalyzed Society) has announced the winners of its “Grands cross-coupling reactions.[3b] Knochel is on the Prix” and “Prix binationaux”. We feature the Editorial Board of ChemPlusChem and the Inter- awardees here. national Advisory Board of Chemistry—An Asian J. Antoine Baceiredo (Laboratoire Heterochi- Journal. mie Fondamentale et AppliquØe(LHFA), Univer- Zhigang Shuai (Tsinghua University) has been sitØ Paul Sabatier,Toulouse) is the recipient of the honored with the Prix franco-chinois.Shuai studied Grand Prix Joseph-Achille Le Bel. Baceiredo stud- at Sun Yat-sen University,Guangzhou, and Jinan ied at the UniversitØ Paul Sabatier,where he University,Guangzhou, and worked with Xin Sun completed his PhD (supervised by Guy Bertrand) at Fudan University,Shanghai, for his PhD (com- in 1982. From 1985–1986, he was apostdoctoral pleted in 1989). From 1990–2001, he was apost- researcher with William P. Weber at the University doctoral researcher with Jean-Luc BrØdas at the of Southern California. He subsequently joined the UniversitØ de Mons,and from 2001–2008, he was CNRS,and in 1992, he was made Directeur de research professor at the Insitute of Chemistry, Recherche at the Laboratoire de Chimie de Chinese Academy of Sciences.Hewas made Coordination, Toulouse.Hejoined at the LHFA Changjiang