Urethral Discharge
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

American Materia Medica, Therapeutics and Pharmacognosy
American Materia Medica, Therapeutics and Pharmacognosy Developing the Latest Acquired Knowledge of Drugs, and Especially of the Direct Action of Single Drugs Upon Exact Conditions of Disease, with Especial Reference of the Therapeutics of the Plant Drugs of the Americas. By FINLEY ELLINGWOOD, M.D. 1919 Late Professor of Materia Medica and Therapeutics in Bennett Medical College, Chicago; Professor of Chemistry in Bennett Medical College 1884-1898; Author, and Editor of Ellingwood's Therapeutist; Member National Eclectic Medical Association; American Medical Editors' Association. Abridged to include only the botanical entries, and arranged in alphabetical order by latin names Southwest School of Botanical Medicine P.O. Box 4565, Bisbee, AZ 85603 www.swsbm.com ABIES. Abies canadensis Synonym—Hemlock spruce. CONSTITUENTS— Tannic acid, resin, volatile oil. Canada pitch, or gum hemlock, is the prepared concrete juice of the pinus canadensis. The juice exudes from the tree, and is collected by boiling the bark in water, or boiling the hemlock knots, which are rich in resin. It is composed of one or more resins, and a minute quantity of volatile oil. Canada pitch of commerce is in reddish-brown, brittle masses, of a faint odor, and slight taste. Oil of hemlock is obtained by distilling the branches with water. It is a volatile liquid, having a terebinthinate odor and taste. PREPARATIONS— Canada Pitch Plaster Tincture of the fresh hemlock boughs Tincture of the fresh inner bark. Specific Medicine Pinus. Dose, from five to sixty minims. The hemlock spruce produces three medicines; the gum, used in the form of a plaster as a rubifacient in rheumatism and kindred complaints; the volatile oil—oil of hemlock—or a tincture of the fresh boughs, used as a diuretic in diseases of the urinary organs, and wherever a terebinthinate remedy is indicated; and a tincture of the fresh inner bark, an astringent with specific properties, used locally, and internally in catarrh. -

Case Report 4 Colovesicular Fistula
Case Report 4 Colovesicular Fistula Daniele Lauretta Agius & Caroline Attard Reviewed by: Mr. Dennis T. Gatt LRCP(London) FRCS(England) FRCS(Edinburgh) IOM Summary A fistula is an atypical connection between two epithelial surfaces, in the case of an enterovesical fistula between the urinary and gastrointestinal systems. These may be the result of a number of causes including: 1. Congenital abnormalities 2. Inflammatory diseases of the bowel (such as diverticulitis and Crohn’s Disease) 3. Cancer 4. Infection 5. Trauma 6. Iatrogenic (such as a post-operative complication) [3] A colovesical fistula (colovesicular fistula), an abnormal connection between the bladder and colon, is a known complication of diverticular disease, occurring in around 2%-22% of patients suffering from diverticulosis. These fistulae tend to occur three times more often in males than in females. The difference in occurrence is thought to be related to the fact that in females there is the uterus which may prevent the colon and bladder from coming into contact with each other. In fact in females other types of fistulae, such as vesicovaginal and enterovaginal, occur more frequently than colovesical fistulae. [2] Aim: This article highlights the importance of the early identification and management of colovesical fistulae, which although uncom- mon complications of diverticulitis, can be very uncomfortable for the patient and if not treated early, can lead to high morbidity. Presenting Complaint: A 58- year-old gentleman was admitted to hospital for an elective sigmoidectomy in view of a colovesical fistula. The patient had been diagnosed with a colovesical fistula two months prior to the surgical operation. -

List of CPRD Medcodes Used to Define Clinical Features
S1: List of CPRD Medcodes used to define 3469 Chronic cystitis unspecified clinical features 4498 [D]Painful urination 5350 [D]Dysuria Medcode Description 7014 C/O ‐ ureteric pain 7579 Suspected UTI Visible haematuria 8433 Urethral pain 6901 Clot haematuria 9378 Recurrent urinary tract infections 7232 Frank haematuria 10295 Acute gonococcal cystitis 9651 Painless haematuria 10515 Recurrent urinary tract infection 35555 Urine: red ‐ blood 10857 Other specified cystitis Non‐visible haematuria 11315 Other chronic cystitis NOS 2784 Microscopic haematuria 12484 Cystitis NOS 13915 RBCs‐ red blood cells in urine 12570 Post operative urinary tract infection 13919 Urine: trace non‐haemol. blood UTI ‐ urinary tract infection in 13929 Urine blood test = +++ 14644 pregnancy 13932 Urine: trace haemolysed blood 15074 Acute cystitis 13934 Urine blood test = + 15599 [D]Dysuria NOS 19792 Urine blood test = ++ Urethral and urinary tract disorders 15787 NOS 29463 Urine microscopy:RBC's present 22682 Cystitis cystica Non‐specific haematuria 29497 Prostatocystitis 507 Haematuria 30068 Subacute cystitis 6030 Haematuria ‐ symptom 32787 Other chronic cystitis 6234 Blood in urine ‐ symptom 34630 Other cystitis NOS 6659 Blood in urine ‐ haematuria Recurrent benign haematuria 38572 Xanthogranulomatous pyelonephritis 7164 syndrome 38698 Acute pyelonephritis NOS 17060 Recurrent and persistent haematuria 48908 Chronic gonococcal cystitis 19361 Traumatic haematuria 49235 Tuberculous pyelonephritis 20357 Painful haematuria 52859 [X]Painful micturition, unspecified Recurrent -

HPSM Incontinence Supply Policy
HPSM Incontinence Supply Policy Effective June 1, 2017, the prior authorization (PA) requirements for incontinence supplies for all Health Plan of San Mateo (HPSM) members (including California Children’s Services members) will be changing. The new incontinence supply policy is outlined below. This policy can also be found on the HPSM website at: https://www.hpsm.org/providers/authorizations.aspx Prior Authorization Requirements By HCPCS Codes Table 1 provides information on the prior authorization requirement by HCPCS code. Table 1. Incontinence Supply HCPCS Codes Code Code Description Prior Authorization Comments Required? A4335 IC supply, misc (Washes) These codes will not Members can choose either washes require prior authorization or wipes. Both products cannot be when they meet all the used at the same time. A4554 Disposable underpads all sizes following requirements: A6250 Skin sealnt protct moisutrzr Product must be on ointment the HPSM T4521 Adult disposable incont brief/diaper SM Incontinence Supply T4522 Adult disposable incont brief/diaper Formulary. The MD Formulary can be T4523 Adult disposable incont brief/diaper found at: LG https://www.hpsm.org/ T4524 Adult disposable incont brief/diaper providers/authorization XLG s.aspx T4525 Adult disposable incont underwear SM Patient must also have T4526 Adult disposable incont underwear a primary diagnosis MD that is the cause of T4527 Adult disposable incont underwear incontinence LG Patient must have an T4528 Adult disposable incont underwear incontinence diagnosis XLG code -

Bladder Endometriosis; an Under Diagnosed Dec 2016; Published Date: 03 Feb 2016
ISSN 2470-0991 SciForschenOpen HUB for Scientific Research Journal of Surgery: Open Access Case Report Volume: 2.2 Open Access Received date: 07 Dec 2015; Accepted date: 28 Bladder Endometriosis; An Under Diagnosed Dec 2016; Published date: 03 Feb 2016. cause of Bladder Pain Syndrome: Report of Citation: Barrabino R, Fernández-Sánchez A, Gil- Julio H (2016) Bladder Endometriosis; An Under Diagnosed cause of Bladder Pain Syndrome: Two New Cases Managed with Endoscopic Report of Two New Cases Managed with Endoscopic Resection. J Surg Open Access 2(2): Resection doi http://dx.doi.org/10.16966/2470-0991.114 Rocio Barrabino1, Antonio Fernández-Sánchez1 and Hernani Gil-Julio2* Copyright: © 2016 Barrabino R, et al. This is an open-access article distributed under the terms 1Department of Urology, Complejo Hospitalario de Granada. Granada, Spain of the Creative Commons Attribution License, 2Urology Service, Hospital Don Benito-Villanueva. Don Benito, Spain which permits unrestricted use, distribution, and reproduction in any medium, provided the original * Corresponding author: Hernani Gil Julio, Servicio de Urología, Hospital Don Benito, Ctra. author and source are credited. Don Benito-Villanueva, Km 3 – 06400, Don Benito, Spain, Tel: +34 924 38 68 00; E-mail: [email protected] Abstract Endometriosis is a severely debilitating disease which affects primarily women of reproductive age. Endometriotic nodules involving the urinary tract is less common and can occur in 1–2% of all patients with endometriosis, and affects the bladder in 90% of these cases. In this article we report two cases of bladder endometriosis in two young females who were referred for macroscopic haematuria and the finding of exophytic intravesical lesions by transabdominal ultrasound imaging. -

The Eclectic Materia Medica, Pharmacology and Therapeutics by Harvey Wickes Felter, M.D
monographs extracted from The Eclectic Materia Medica, Pharmacology and Therapeutics by Harvey Wickes Felter, M.D. (1922) NOTE: Throughout these monographs are references to “Specific Medicines”. In some respects Specific Medicines are the single reason that Eclecticism survived so long in the face of “Organized Medicine” and were still being manufactured for the surviving Eclectic M.D.s as late as the early 1960s. Using up to eight organic solvents and the Lloyd Extractor, Specific Medicines represented the strongest possible concentration of the bioactive aspects of botanicals that would stay in a colloidal solution. Perfected over four decades by John Uri Lloyd, each Specific Medicine was prepared according to the nature of THAT specific plant. You cannot translate a Specific Medicine into “tincture” or “fluidextract”. The latter are GENERIC or standard strengths applied across the board to ALL botanicals. A Specific Medicine represented the greatest strength, without degradation, for a PARTICULAR plant, using anywhere from several to all of the solvents to achieve this. The Eclectic physician was trained to use botanicals in an oftentimes rural setting, and these medicines had to resist breakdown in the deepest winter and the hottest summer. Since they needed to contain even the most ephemeral constituents of a plant remedy, Lloyd approached each plant separately. The amazing quality of these preparations assuredly maintained the Eclectic Movement long after others had faded. Lloyd’s recipes were Patent Medicines, were not “official”, and when relatives finally closed down the Lloyd Brother’s Pharmacy in Cincinnati, these formulae disappeared. One of the hottest topics for many years amongst professional herbalists in North America and Europe has been “So who has the Lloyd Formulas, already?” Since we cannot access them, the best approach is the use of well made tinctures, capsules or tea. -
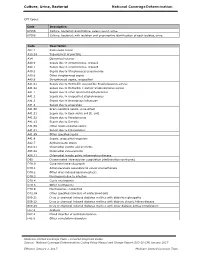
Culture, Urine, Bacterial National Coverage Determination
Culture, Urine, Bacterial National Coverage Determination CPT Codes: Code Description 87086 Culture, bacterial; quantitative, colony count, urine. 87088 Culture, bacterial; with isolation and presumptive identification of each isolates, urine. Code Description A02.1 Salmonella sepsis A18.14 Tuberculosis of prostate A34 Obstetrical tetanus A40.0 Sepsis due to streptococcus, group A A40.1 Sepsis due to streptococcus, group B A40.3 Sepsis due to Streptococcus pneumoniae A40.8 Other streptococcal sepsis A40.9 Streptococcal sepsis, unspecified A41.01 Sepsis due to Methicillin susceptible Staphylococcus aureus A41.02 Sepsis due to Methicillin resistant Staphylococcus aureus A41.1 Sepsis due to other specified staphylococcus A41.2 Sepsis due to unspecified staphylococcus A41.3 Sepsis due to Hemophilus influenzae A41.4 Sepsis due to anaerobes A41.50 Gram-negative sepsis, unspecified A41.51 Sepsis due to Escherichia coli [E. coli] A41.52 Sepsis due to Pseudomonas A41.53 Sepsis due to Serratia A41.59 Other Gram-negative sepsis A41.81 Sepsis due to Enterococcus A41.89 Other specified sepsis A41.9 Sepsis, unspecified organism A42.7 Actinomycotic sepsis A56.01 Chlamydial cystitis and urethritis A56.02 Chlamydial vulvovaginitis A56.11 Chlamydial female pelvic inflammatory disease D65 Disseminated intravascular coagulation [defibrination syndrome] D70.0 Congenital agranulocytosis D70.1 Agranulocytosis secondary to cancer chemotherapy D70.2 Other drug-induced agranulocytosis D70.3 Neutropenia due to infection D70.4 Cyclic neutropenia D70.8 Other neutropenia -

Common Lesions of the Urethra in Women CARL E
February, 1952 69 Common Lesions of the Urethra in Women CARL E. BURKLAND, M.D., Sacramento Lesions of the urethra should always be consid- SUMMARY ered a possible source of abdominal discomfort in Urethral disease in women and women. Recognition of them is important not only girls often because of the distress they cause in themselves and is overlooked. As the urine may seem to be the erroneous normal as determined by repeated urinalysis, diagnoses and needless procedures the symptoms-urinary frequency and burn- that may result if they are overlooked, but also be- ing-may be attributed entirely to other pel- cause of the back-pressure effects upon the upper vic disease or to functional disorder. Since urinary tract. Urethritis in young girls'0 particu- erroneous diagnosis may lead to unnecessary larly has never been given the emphasis merited by procedures or to neglect of treatment with the incidence and importance of the disease. Too consequent development of severe disease in often in girls pyelitis is erroneously diagnosed and the kidneys or ureters, it is important to con- chronic urethritis overlooked or improperly evalu- sider urethral lesions as a possible cause in ated until more serious disease results from it. any case of abdominal discomfort in women. The most common urethral diseases in women are The most common lesions of the urethra in acute and chronic urethritis, stricture, caruncle, in- women are urethritis, stricture, caruncle, in- flammatory polyps and cysts in the posterior urethra flammatory polyps and cysts, prolapse of the and in the neck of the bladder, prolapse of the urethra, and diverticulum. -

Hospital Visits After Urology Ambulatory Surgical Center Procedures (Version 1.0) Measure Technical Report
Hospital Visits after Urology Ambulatory Surgical Center Procedures (Version 1.0) Measure Technical Report Submitted by: Yale New Haven Health Services Corporation – Center for Outcomes Research and Evaluation (CORE) Prepared for: Centers for Medicare & Medicaid Services (CMS) May 2017 Table of Contents List of Tables ................................................................................................................................... 4 List of Figures .................................................................................................................................. 5 Yale New Haven Health Services Corporation – Center for Outcomes Research and Evaluation (CORE) Project Team ....................................................................................................................... 6 Acknowledgements ......................................................................................................................... 7 Executive Summary ................................................................................................................. 9 1.1 Rationale for Assessing Hospital Visits after Ambulatory Surgery ................................. 9 1.2 Measure Development ................................................................................................. 10 1.3 Measure Specifications ................................................................................................. 10 1.4 Distribution of Measure Scores ................................................................................... -

Outstanding Symptoms Which Suggest That the Bladder Is Not Being Completely Emptied
DECEMBER., 1944 MICTURITION : RETENTION 340 Postgrad Med J: first published as 10.1136/pgmj.20.229.340 on 1 December 1944. Downloaded from ACUTE AND CHRONIC RETENTION By ARTHUR JACOBS, F.R.F.P.S.Glasg. (Surgeon, Urological Dept., Glasgow Royal Infirmary) By acute retention is meant a sudden and complete inability to pass urine. A chronic retention means that the patient is unable to empty the bladder, though micturition is still possible, and may even be apparently quite free. The quantity unevacuated from the bladder is termed residual urine, and this may vary in amount from a few ounces in cases of minor obstruction, to two or more pints in advanced manifestations. The two phases may merge from one to the other. Thus an acute retention may supervene on a previously established chronic retention and revert back to the latter category following relief of the acute attack. Whilst the diagnosis -of acute retention is self-evident, that of chronic retention may not, at first, be apparent. Diminution in the force of the stream and urinary frequency are the outstanding symptoms which suggest that the bladder is not being completely emptied. Supra- pubic palpation and percussion may confirm the diagnosis providing the abdominal wall is not too obese. Confirmation is definitely obtained by passing a catheter, after the bladder has been emptied to the best of the patient's ability. If a large residual is present, not more than eight to ten ounces should be allowed to run off at such a diagnostic test. It should be borne in mind that advice is frequently sought from patients with a chronic vesical distension, who complain of little or no disturbance directly referable to the bladder. -

Rapid Recurrence of Giant Multilocular Prostatic Cystadenoma After Laparoscopic Excision for Primary Case: a Case Report
medicina Case Report Rapid Recurrence of Giant Multilocular Prostatic Cystadenoma after Laparoscopic Excision for Primary Case: A Case Report Tae-Soo Choi, Dong-Gi Lee, Koo-Han Yoo and Gyeong-Eun Min * Department of Urology, College of Medicine, Kyung Hee University, Seoul 05278, Korea; [email protected] (T.-S.C.); [email protected] (D.-G.L.); [email protected] (K.-H.Y.) * Correspondence: [email protected]; Tel.: +82-2-440-7735; Fax: +82-2-440-7744 Abstract: Giant multilocular prostatic cystadenoma is a rare benign tumor of the prostate gland that presents as a large retroperitoneal pelvic mass. The mass is usually located between the urinary bladder and rectum, and results in obstructive voiding symptoms and a change in bowel habits. Complete surgical excision is the treatment of choice. We present a case of rapid recurrent giant multilocular prostatic cystadenoma after laparoscopic excision for primary case. A previously healthy 54-year-old man presented with acute urinary retention. Prostate MRI showed a large cystic mass approximately 13 cm in size, multiple septa and lobulation in the prostate, and no visible solid lesions. Laparoscopic marsupialization of giant multilocular prostatic cystadenoma cysts was performed. One year later, the patient presented with local recurrence. Repeated laparoscopic complete resection was performed without any complications and further recurrence. Giant multilocular prostatic cystadenoma has the risk of recurrence in case of incomplete resection. Surgical treatment should be performed with the goal of complete removal following the same principles as cancer surgery. Citation: Choi, T.-S.; Lee, D.-G.; Yoo, Keywords: prostate; cystadenoma; laparoscopy; surgery K.-H.; Min, G.-E. -

Original Article Holmium Laser Enucleation of the Prostate Prevents Postoperative Stress Incontinence in Patients with Benign Prostate Hyperplasia
Int J Clin Exp Med 2018;11(3):2572-2576 www.ijcem.com /ISSN:1940-5901/IJCEM0054035 Original Article Holmium laser enucleation of the prostate prevents postoperative stress incontinence in patients with benign prostate hyperplasia Wenhui Song, Jianhui Wu, Junwei Gai, Zhanpo Yang, Shiqiang Yang, Qingtong Ma, Hongshun Ma Department of Urinary Surgery, Tianjin First Center Hospital, Tianjin 300192, China Received August 29, 2016; Accepted December 15, 2017; Epub March 15, 2018; Published March 30, 2018 Abstract: Objective: We aimed to compare the safety and efficacy of the transurethral electrovaporization resec- tion of the prostate (TUEVP) and holmium laser enucleation of the prostate (HoLEP) in treating BPH, especially in preventing the postoperative stress urinary incontinence, which has been rarely studied. Methods: A total of 100 patients with BPH admitted in our department from August 2013 to October 2015 were prospectively enrolled and randomly divided into two groups who underwent TUEVP (n = 50) or HoLEP (n = 50), respectively. The demographic and clinical characteristics including the procedural parameters and hospitalization duration were collected and analyzed. Results: HoLEP had advantages over TUVEP, which was evidenced by the statistically significant differ- ences found on less operative duration (P<0.01), intraoperative blood loss (P<0.01) and resected prostate weight (P<0.01), intraoperative bladder irrigation duration (P<0.05), urinary catheter indwelling time (P<0.05), postopera- tive blood loss (P<0.05) and hospitalization duration (P<0.05) between the two groups. The 2-week and 3-month incidence of stress incontinence in HoLEP group were both lower than those in TUEVP group (P<0.05), while there was no significant difference on the IPSS and Qmax at 3-month follow-up after the surgery (P>0.05).