Common Drug Classes, Drug-Nutrient Depletions, & Drug
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Does Dietary Fiber Affect the Levels of Nutritional Components After Feed Formulation?
fibers Article Does Dietary Fiber Affect the Levels of Nutritional Components after Feed Formulation? Seidu Adams 1 ID , Cornelius Tlotliso Sello 2, Gui-Xin Qin 1,3,4, Dongsheng Che 1,3,4,* and Rui Han 1,3,4 1 College of Animal Science and Technology, Jilin Agricultural University, Changchun 130118, China; [email protected] (S.A.); [email protected] (G.-X.Q.); [email protected] (R.H.) 2 College of Animal Science and Technology, Department of Animal Genetics, Breeding and Reproduction, Jilin Agricultural University, Changchun 130118, China; [email protected] 3 Key Laboratory of Animal Production, Product Quality and Security, Jilin Agricultural University, Ministry of Education, Changchun 130118, China 4 Jilin Provincial Key Laboratory of Animal Nutrition and Feed Science, Jilin Agricultural University, Changchun 130118, China * Correspondence: [email protected]; Tel.: +86-136-4431-9554 Received: 12 January 2018; Accepted: 25 April 2018; Published: 7 May 2018 Abstract: Studies on dietary fiber and nutrient bioavailability have gained an increasing interest in both human and animal nutrition. Questions are increasingly being asked regarding the faith of nutrient components such as proteins, minerals, vitamins, and lipids after feed formulation. The aim of this review is to evaluate the evidence with the perspective of fiber usage in feed formulation. The consumption of dietary fiber may affect the absorption of nutrients in different ways. The physicochemical factors of dietary fiber, such as fermentation, bulking ability, binding ability, viscosity and gel formation, water-holding capacity and solubility affect nutrient absorption. The dietary fiber intake influences the different methods in which nutrients are absorbed. -

Super Green Superfoods
[Plant-Based Ingredients] Vol. 17 No. 12 December 2012 Super Green Superfoods By Celeste Sepessy, Associate Editor Once the secret of Birkenstock-wearing progressives, green foods are now filling the shopping carts of informed—if not guilty—conventional consumers. Nutrient-dense greens date back millions of years, and humans in the know have been eating them for centuries; green foods manufacturers commonly tout their ingredients as the energy food of ancient Mesoamericans, namely the Aztecs. The term "green foods" encompasses a range of raw materials including algae (chlorella, spirulina, etc.), grasses (alfalfa, barley grass, wheat grass, etc.) and common green vegetables (broccoli, spinach, etc.). Though each ingredient boasts its own benefits, they all pack a well-rounded nutritional punch not often found elsewhere. "Spirulina is nature's multivitamin," said John Blanco, president of AnMar International, noting the microalgae has 60-percent protein, unsaturated fatty acids and vitamin precursors, such as amino acids and proenzymes. "It's not a complete 100-percent balanced vitamin tablet, but it's pretty close." And this nutritional breakdown is similar across the green board, as the ingredients are densely filled with phytonutrients, antioxidants, vitamins, minerals and nucleic acid, among other nutrients. Consumers of all demographics are becoming more aware of the benefits of eating these green superfoods; Guinevere Lynn, director of business development at Sun Chlorella, pointed to the media for the industry's popularity surge. "Mass media has certainly played a major role in this 'green renaissance,' " she explained, citing Dr. Oz's help in particular. The television medical personality is a huge proponent of green foods, and Dr. -

Drug Interactions: a Napdi Center Recommended Approach S
Supplemental material to this article can be found at: http://dmd.aspetjournals.org/content/suppl/2018/05/07/dmd.118.081273.DC1 1521-009X/46/7/1046–1052$35.00 https://doi.org/10.1124/dmd.118.081273 DRUG METABOLISM AND DISPOSITION Drug Metab Dispos 46:1046–1052, July 2018 Copyright ª 2018 by The American Society for Pharmacology and Experimental Therapeutics Perspective Selection of Priority Natural Products for Evaluation as Potential Precipitants of Natural Product–Drug Interactions: A NaPDI Center Recommended Approach s Emily J. Johnson,1 Vanessa González-Peréz,2 Dan-Dan Tian, Yvonne S. Lin, Jashvant D. Unadkat, Allan E. Rettie, Danny D. Shen, Jeannine S. McCune, and Mary F. Paine Center of Excellence for Natural Product Drug Interaction Research, Spokane, Washington (Y.S.L., J.D.U., A.E.R., D.D.S., J.S.M., M.F.P.); Department of Pharmaceutical Sciences, Washington State University, Spokane, Washington (E.J.J., V.G.-P., D.-D.T., Downloaded from M.F.P.); Department of Pharmaceutics (Y.S.L., J.D.U., D.D.S., J.S.M.) and Department of Medicinal Chemistry (A.E.R.), University of Washington, Seattle, Washington; and Department of Population Sciences, City of Hope, Duarte, California (J.S.M.) Received March 2, 2018; accepted May 3, 2018 dmd.aspetjournals.org ABSTRACT Pharmacokinetic interactions between natural products (NPs) and pharmacokinetic NPDIs. Guided information-gathering tools were used conventional medications (prescription and nonprescription) are a long- to score, rank, and triage NPs from an initial list of 47 candidates. standing but understudied problem in contemporary pharmacotherapy. -

ADVISORY BOARDS Each Issue of Herbaigram Is Peer Reviewed by Various Members of Our Advisory Boards Prior to Publication
ADVISORY BOARDS Each issue of HerbaiGram is peer reviewed by various members of our Advisory Boards prior to publication . American Botanical Council Herb Research Dennis V. C. Awang, Ph.D., F.C.I.C., MediPiont Natural Gail B. Mahadr, Ph.D., Research Assistant Professor, Products Consul~ng Services, Ottowa, Ontario, Conodo Deportment o Medical Chemistry &Pharmacognosy, College of Foundation Pharmacy, University of Illinois, Chicago, Illinois Manuel F. Balandrin, R.Ph., Ph.D., Research Scien~st, NPS Rob McCaleb, President Pharmaceuticals, Salt LakeCity , Utah Robin J. Maries, Ph.D., Associate Professor of Botany, Brandon University, Brandon, Manitoba, Conodo Mi(hael J. Balidt, Ph.D., Director of the lns~tute of Econom ic Glenn Appelt, Ph.D., R.Ph., Author and Profess or Botany, the New York Botanical Gorden, Bronx, New York Dennis J. M(Kenna, Ph.D., Consulting Ethnophormocologist, Emeritus, University of Colorado, and with Boulder Beach Joseph M. Betz, Ph.D., Research Chemist, Center for Food Minneapolis, Minnesota Consulting Group Safety and Applied Nutri~on, Division of Natural Products, Food Daniel E. Moerman, Ph.D., William E. Stirton Professor of John A. Beutler, Ph.D., Natural Products Chemist, and Drug Administro~on , Washington, D.C. Anthropology, University of Michigon/ Deorbom, Dearborn, Michigan Notional Cancer Institute Donald J. Brown, N.D., Director, Natural Products Research Consultants; Faculty, Bastyr University, Seattle, Washington Samuel W. Page, Ph.D., Director, Division of Natural Products, Robert A. Bye, Jr., Ph.D., Professor of Ethnobotony, Notional University of Mexico Thomas J. Carlson, M.S., M.D., Senior Director, Center for Food Safety and Applied Nutri~on , Food and Drug Administro~on , Washington, D.C. -

Selective Mtorc2 Inhibitor Therapeutically Blocks Breast Cancer Cell Growth and Survival
Author Manuscript Published OnlineFirst on January 22, 2018; DOI: 10.1158/0008-5472.CAN-17-2388 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Selective mTORC2 inhibitor therapeutically blocks breast cancer cell growth and survival Thomas A. Werfel1, 3, Shan Wang2, Meredith A. Jackson1, Taylor E. Kavanaugh1, Meghan Morrison Joly3, Linus H. Lee1, Donna J. Hicks3, Violeta Sanchez4, Paula Gonzalez Ericsson4, Kameron V. Kilchrist1, Somtochukwu C. Dimobi1, Samantha M. Sarett1, Dana Brantley-Sieders2, Rebecca S. Cook1,3,4* and Craig L. Duvall1* 1Department of Biomedical Engineering, Vanderbilt University, Nashville, TN 37232 USA 2Department of Medicine, Vanderbilt University Medical Center, Nashville, TN 37232.USA 3Department of Cell and Developmental Biology, Vanderbilt University School of Medicine, Nashville, TN 37232 USA 4Breast Cancer Research Program, Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN 37232 USA Running Title: A selective mTORC2 inhibitor blocks breast cancer growth Key Words: Breast Cancer, mTOR, Rictor, RNA interference, Nanomedicine *To whom correspondence should be addressed: Craig L. Duvall, PhD Vanderbilt University School of Engineering Department of Biomedical Engineering Nashville, TN 37232 Phone: (615) 322-3598 Fax: (615) 343-7919 Email: [email protected] Rebecca S. Cook, PhD Vanderbilt University Medical Center Department of Cancer Biology Nashville, TN 37232 Phone: (615) 936-3813 Fax: (615) 936-3811 Email: [email protected] Funding. This work was supported by Specialized Program of Research Excellence (SPORE) grant NIH P50 CA098131 (VICC), Cancer Center Support grant NIH P30 CA68485 (VICC), NIH F31 CA195989-01 (MMW), NIH R01 EB019409, DOD CDMRP OR130302, NSF GFRP 1445197, and CTSA UL1TR000445 from the National Center for Advancing Translational Sciences. -

Angiotensin-Converting Enzyme (ACE) Inhibitors Single Entity Agents
Therapeutic Class Overview Angiotensin-Converting Enzyme (ACE) Inhibitors Single Entity Agents Therapeutic Class Overview/Summary: The renin-angiotensin-aldosterone system (RAAS) is the most important component in the homeostatic regulation of blood pressure.1,2 Excessive activity of the RAAS may lead to hypertension and disorders of fluid and electrolyte imbalance.3 Renin catalyzes the conversion of angiotensinogen to angiotensin I. Angiotensin I is then cleaved to angiotensin II by angiotensin- converting enzyme (ACE). Angiotensin II may also be generated through other pathways (angiotensin I convertase).1 Angiotensin II can increase blood pressure by direct vasoconstriction and through actions on the brain and autonomic nervous system.1,3 In addition, angiotensin II stimulates aldosterone synthesis from the adrenal cortex, leading to sodium and water reabsorption. Angiotensin II exerts other detrimental cardiovascular effects including ventricular hypertrophy, remodeling and myocyte apoptosis.1,2 The ACE inhibitors block the conversion of angiotensin I to angiotensin II, and also inhibit the breakdown of bradykinin, a potent vasodilator.4 Evidence-based guidelines recognize the important role that ACE inhibitors play in the treatment of hypertension and other cardiovascular and renal diseases. With the exception of Epaned® (enalapril solution) and Qbrelis® (lisinopril solution), all of the ACE inhibitors are available generically. Table 1. Current Medications Available in Therapeutic Class5-19 Generic Food and Drug Administration -

Macronutrients and Human Health for the 21St Century
nutrients Editorial Macronutrients and Human Health for the 21st Century Bernard J. Venn Department of Human Nutrition, University of Otago, Dunedin 9054, New Zealand; [email protected] Received: 30 July 2020; Accepted: 4 August 2020; Published: 7 August 2020 Abstract: Fat, protein and carbohydrate are essential macronutrients. Various organisations have made recommendations as to the energy contribution that each of these components makes to our overall diet. The extent of food refining and the ability of food systems to support future populations may also impact on how macronutrients contribute to our diet. In this Special Issue, we are calling for manuscripts from all disciplines to provide a broad-ranging discussion on macronutrients and health from personal, public and planetary perspectives. Keywords: macronutrient; fat; protein; carbohydrate; acceptable macronutrient distribution range; starch; sustainability The macronutrients, fat, protein and carbohydrate provide energy and essential components to sustain life. Fat is composed of glycerol and fatty acids; protein is an agglomeration of amino acids; and carbohydrate is simple sugars occurring either as monosaccharides or chains of connected monosaccharides (e.g., starch) whose bonds are either hydrolysed in the human small intestine to monosaccharides or are resistant to hydrolysis (dietary fibre). To maintain longevity and health, a combination of these macronutrients is required in our diet. It is elusive as to whether there is a combination of macronutrients that provides optimal health. When expressed as a percentage of energy to the diet, human populations have historically survived on diets with greatly differing proportions of these macronutrients. For example, the animal-based diet of an Alaskan Inuit group was found to comprise 33% protein, 41% fat and 26% carbohydrate [1]. -
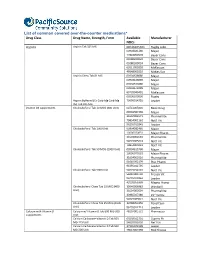
List of Common Covered Over-The-Counter Medications*
List of common covered over-the-counter medications* Drug Class Drug Name, Strength, Form Available Manufacturer NDCs Aspirin Aspirin Tab 325 MG 00536105301 Rugby Labo 00904681180 Major 12843054578 Bayer Cons 00280200020 Bayer Cons 00280200024 Bayer Cons 62011002003 McKesson 49348000110 McKes Sun Aspirin Chew Tab 81 MG 00904628880 Major 00904628889 Major 00904679480 Major 00904679489 Major 63739043401 McKesson 00536100836 Rugby Aspirin Buffered (Ca Carb-Mg Carb-Mg 70000014701 Leader Ox) Tab 325 MG Vitamin D3 supplements Cholecalciferol Tab 10 MCG (400 Unit) 00761005820 Basic Drug 00904582360 Major 31604002671 PharmaVite 79854001162 Nat’l Vit 96295012845 Leader Cholecalciferol Tab 1000 Unit 00904582460 Major 10006070033 Major Pharm 31604002683 PharmaVite 54629005024 Nat’l Vit 79854005023 Nat’l Vit Cholecalciferol Tab 50 MCG (2000 Unit) 00904615760 Major 10006070162 Major Pharm 31604002516 PharmaVite 51645092199 Plus Pharm 96295012795 Leader Cholecalciferol Tab 5000 Unit 54629794101 Nat’l Vit 58487003702 Freeda Vit 96295012846 Leader 43292056338 Magno-Hump Cholecalciferol Chew Tab 10 MCG (400 35046006982 Windwill Unit) 31604002604 PharmaVite 40985027380 21st Centu 54629089821 Nat’l Vit Cholecalciferol Chew Tab 25 MCG (1000 30768050356 Rexall Sun Unit) 96295012713 Leader Calcium with Vitamin D Calcium w/ Vitamin D Tab 600 MG-200 48107005122 Pharmassur supplements Unit Calcium Carbonate-Vitamin D Tab 500 60258012701 Cypress Ph MG-125 Unit 54629031630 Nat’l Vit Calcium Carbonate-Vitamin D Tab 500 37205043198 Leader MG-200 Unit 49614067298 -

Is Alcohol Use Related to High Cholesterol in Premenopausal
Research iMedPub Journals Journal of Preventive Medicine 2018 http://www.imedpub.com/ Vol.3 No.1:3 ISSN 2572-5483 DOI: 10.21767/2572-5483.100024 Is Alcohol Use Related To High Cholesterol in Premenopausal Women Aged 40-51 Years Old? Sydnee Homeyer, Jessica L Hartos*, Holli Lueg, Jessica Moore and Patricia Stafford Department of Physician Assistant Studies, University of North Texas Health Science Center, USA *Corresponding author: Jessica L. Hartos, Department of Physician Assistant Studies, University of North Texas Health Science Center, USA, Tel: 817-735-2454; Fax: 817-735-2529; E-mail: [email protected] Received date: November 13, 2017; Accepted date: January 8, 2018; Publication date: January 15, 2018 Citation: Homeyer S, Hartos JL, Lueg H, Moore J, Stafford P, et al. (2018) Is Alcohol Use Related To High Cholesterol In Premenopausal Women Aged 40-51 Years Old? J Prev Med Vol.3 No.3: 3. Copyright: © 2018 Homeyer S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited. Introduction Abstract Increased serum cholesterol levels is a major risk factor for coronary heart disease (CHD) [1], and over seventy-one million Purpose: Alcohol use and cholesterol are related in men and American adults have high cholesterol (27%) [2,3]. In American postmenopausal women but relations between alcohol use women, cholesterol levels have been shown to increase with and cholesterol are unclear for premenopausal women. The age, and nearly 1 in 2 American women has high or borderline purpose of this study was to determine whether alcohol use was related to cholesterol in women aged 40-51 years old. -

E the Effect of Topiramate on Weight Loss in Patients with Type 2 Diabetes
E The effect of topiramate on weight loss in patients with type 2 diabetes RTICL Sedighe Moradi, Scott Reza Jafarian Kerman, Mina Mollabashi A Institute of Endocrinology and Metabolism (Hemmat Campus), Firoozgar Hospital, Tehran University of Medical Sciences, Tehran, Iran Background: Obesity has been associated with several co‑morbidities such as diabetes and increased mortality. In general, the use of medication promotes only a modest weight loss in the range of 2 to 10 kg, usually most effective during the first 6 months of therapy; however, studies have shown positive effects on other risk factors such as blood pressure and serum glucose levels, but there are fewer studies in patients with diabetes. The aim of this study was to assess the effect of topiramate on weight reduction patients with type 2 diabetes. Materials and Methods: This was a 32‑week randomized clinical trial study of 69 subjects during 2008‑2010. RIGINAL Patients, in two treatment groups were given topiramate (39 patients) and Placebo (30 patients) and were subjected to participation in a non‑pharmacologic lifestyle intervention program; which were randomly allocated in our two groups. The percentage change in body O weight and Body Mass Index (BMI) at the end of the study was the primary efficacy endpoint and secondary indicators were changes in blood pressure (BP), proportion of subjects who achieved 5% or 10% weight loss, changes in lipid profile (total cholesterol, low‑density lipoprotein cholesterol, high‑density lipoprotein cholesterol, triglycerides); and changes in glycosylated hemoglobin (HgA1c). Paired samples and independent samples t‑test was used for statistical analysis. -

Management of Hyperglycaemia and Steroid (Glucocorticoid) Therapy
Management of Hyperglycaemia and Steroid (Glucocorticoid) Therapy October 2014 This document is coded JBDS 08 in the series of JBDS documents Other JBDS documents: Admissions avoidance and diabetes: guidance for clinical commissioning groups and clinical team; December 2013, JBDS 07 The management of the hyperosmolar hyperglycaemic state (HHS) in adults with diabetes; August 2012, JBDS 06 Glycaemic management during the inpatient enteral feeding of stroke patients with diabetes; June 2012, JBDS 05 Self-management of diabetes in hospital; March 2012, JBDS 04 Management of adults with diabetes undergoing surgery and elective procedures: improving standards; April 2011, JBDS 03 The Management of Diabetic Ketoacidosis in Adults; revised September 2013, JBDS 02 The hospital management of hypoglycaemia in adults with diabetes mellitus; revised September 2013, JBDS 01 These documents are available to download from: ABCD website: www.diabetologists-abcd.org.uk/JBDS/JBDS.htm Diabetes UK website: www.diabetes.org.uk Contents Page Foreword 4 Authorship and acknowledgments 5-6 Introduction 7 Steroids - mechanism of action 8 Steroid therapy – impact on blood glucose 9 Glucose targets 10 Glucose monitoring 11 Diabetes treatment options 12-13 Treatment of steroid induced hyperglycaemia 14-15 Hospital discharge 16-17 Steroid treatment in pregnancy 18 Steroid treatment in end of life 19 Audit standards 20 Controversial areas 21 References 22 Appendix 1 – Algorithm to show treatment of steroid 23 induced diabetes Appendix 2 – Algorithm to show management of patients 24 with diabetes on once daily steroids Appendix 3 – End of life steroid management 25 Appendix 4 – Patient letter – Glucose monitoring and 26 steroid use 3 Foreword This is the latest in the series of Joint British Diabetes Societies for Inpatient Care (JBDS-IP) guidelines, and focuses on steroid induced hyperglycaemia and steroid induced diabetes. -

Effect of Testosterone on the Growth and Virulence of Staphylococcus Aureus
Loyola University Chicago Loyola eCommons Master's Theses Theses and Dissertations 1966 Effect of Testosterone on the Growth and Virulence of Staphylococcus aureus Joseph Lloyd Waner Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_theses Part of the Medicine and Health Sciences Commons Recommended Citation Waner, Joseph Lloyd, "Effect of Testosterone on the Growth and Virulence of Staphylococcus aureus" (1966). Master's Theses. 2065. https://ecommons.luc.edu/luc_theses/2065 This Thesis is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Master's Theses by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1966 Joseph Lloyd Waner Effect of Testosterone on the Growth and Virulence of Staphlococcus aureus By Joseph L. Waner Microbiology Department Stritch School of Medicine A thesis submitted to the Faculty of the Graduate School of Loyola University in partial fulfillment of the requirements for the degree of Master of Science. TABLE OF CONTENTS Lifv--------------------------------------------- ii Abstract-------------------------------------------P. 1 Introd~ction---------------------------------------P. 2-3 ~a terials and Nethod s------------------- ---------------P. 4-10 S. aureus strains------------ -----------------------P. 4 Testosterone Bolutions------------·------·---------P.