Efficiency of Teeth Bleaching After Regenerative Endodontic Treatment: a Systematic Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Tooth Whitening During Orthodontictreatment: a Six-Month
ISSN 2378-7090 SciForschenOpen HUB for Scientific Research International Journal of Dentistry and Oral Health Research Article Volume: 1.4 Open Access Received date: 10 June 2015; Accepted date: 14 Tooth Whitening during Orthodontic July 2015; Published date: 19 July 2015. Treatment: a Six-Month in vitro Assessment Citation: Arboleda-Lopez C, Manasse RJ, Viana G, Bedran-Russo AB, Evans CA (2015) Tooth Whitening during Orthodontic Treatment: a Six- of Effectiveness and Stability Month in vitro Assessment of Effectiveness and Cleidy Arboleda-Lopez, Robert J. Manasse*, Grace Viana, Ana B. Bedran-Russo, Stability. Int J Dent Oral Health 1(4): doi http://dx.doi. and Carla A. Evans org/10.16966/2378-7090.120 Department of Orthodontics, College of Dentistry, University of Illinois at Chicago, USA Copyright: © 2015 Arboleda-Lopez C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, * Corresponding author: Robert J. Manasse, Clinical Associate Professor, College of Dentistry, which permits unrestricted use, distribution, and Department of Orthodontics, University of Illinois at Chicago, Chicago, IL 60612-7211, USA. E-mail: reproduction in any medium, provided the original [email protected] author and source are credited. Introduction was to investigate whether whitening on human teeth with brackets is effective as whitening on human teeth without brackets. Not only are today’s orthodontic patients concerned about the alignment and shape of teeth but also the color; many wish to have their Materials and Methods teeth whitened before the end of orthodontic treatment. Common A sample size of 20 teeth in each group was needed in this study to questions that orthodontists face from patients are, “When can I whiten detect the mean differences in terms of color difference, (∆E) with at least my teeth? Can I whiten my teeth with braces?” The answer often given is 80% power and an error of 5%. -

Illegal Whitening Kit Sales Uncovered
NEWS SCOTTISH GOVERNMENT RECONSIDERS PAY AWARD The British Dental Association (BDA) view of the DDRB’s recommendations had DIARY has persuaded the Scottish Government led it to calculate the increase should be to reconsider the way it is implement- 0.5% on the item of service fees. SEPTEMBER ing this year’s Doctors’ and Dentists’ The SDPC made representations to the Review Body (DDRB) pay award. An Scottish Government that the expenses Aesthetic dentistry for the GDP (Belfast) amended Statement of Dental Remuner- element had already taken into account Date: 17 September 2010 ation is expected to be issued on 1 Octo- by the DDRB in reaching its recommen- Venue: Ramada, Belfast Email: [email protected] ber and payments will be backdated to dation to increase the individual items www.fgdp.org.uk 1 April 2010. on the fee scale and that the Scottish The BDA’s Scottish Dental Practice Com- Government’s approach was there- Fall 2010 American Academy of mittee (SDPC) has successfully argued fore fl awed. The Scottish Government Cosmetic Dentistry International that the DDRB had taken the expenses looked again at the issue and are now Meeting/British Academy of Cosmetic element of the pay award into account able to agree that the uplift for inde- Dentistry Annual Conference and that the 0.9% pay increase should pendent general dental practitioners Date: 23-25 September 2010 apply to the whole item of service rather (GDPs) should be 0.9% applied to the Venue: Hilton London Metropole Hotel than just the expense element of the pay- whole item of service (and not just the Email: [email protected] ment. -

Policy on the Use of Dental Bleaching for Child and Adolescent Patients
ORAL HEALTH POLICIES: USE OF DENTAL BLEACHING Policy on the Use of Dental Bleaching for Child and Adolescent Patients Latest Revision How to Cite: American Academy of Pediatric Dentistry. Policy on 2019 the use of dental bleaching for child and adolescent patients. The Reference Manual of Pediatric Dentistry. Chicago, Ill.: American Academy of Pediatric Dentistry; 2020:112-5. Purpose may vary significantly during the mixed dentition. Full arch The American Academy of Pediatric Dentistry recognizes that cosmetic bleaching during this developmental stage, however, the desire for dental whitening by pediatric and adolescent would result in mismatched dental appearance once the child patients has increased. This policy is intended to help pro- is in the permanent dentition. Adolescents present with fessionals and patients make informed decisions about the unique dental needs, and the impact of tooth discoloration on indications, efficacy, and safety of internal and external bleach- an adolescent’s self-image could be considered an indication ing of primary and young permanent teeth and incorporate for bleaching.8 Tooth whitening has been successful in adoles- such care into a comprehensive treatment plan. cent patients using typical bleaching agents,8 but research is lacking on the effects of bleaching on the primary dentition. Methods Dental whitening may be accomplished by using either This policy was developed by the Council on Clinical Affairs professional or at-home bleaching modalities. Advantages of and adopted in 2004. This document is an update from the in-office whitening or whitening products dispensed and last revision in 2014. This revision included a new literature monitored by a dental professional include: search of the PubMed®/MEDLINE database using the terms: • an initial professional examination to help identify causes dental bleaching, dental whitening, and tooth bleaching; of discoloration and clinical concerns with treatment fields: all; limits: within the last 10 years, humans, English, (e.g., existing restorations, side effects). -

Tooth Whitening Effects on Bracket Bond Strength in Vivo
Original Article Tooth Whitening Effects on Bracket Bond Strength In Vivo Joseph M. Mullinsa; Elizabeth C. Kaob; Chris A. Martinc; Erdogan Guneld; Peter Ngane ABSTRACT Objective: To test the hypothesis that there is no difference between the bracket survival rate of brackets bonded to bleached and unbleached teeth. Materials and Methods: Thirty-eight patients who required comprehensive orthodontic treatment were included in the study. A split mouth technique was used with one arch exposed to in-office whitening gel containing 38% hydrogen peroxide for 30 minutes, while the unbleached arch served as the control. Patients were divided into two groups: Brackets bonded within 24 hours after bleaching and brackets bonded 2–3 weeks after bleaching. The bracket survival rate was com- puted using the log-rank test (Kaplan-Meier Analysis). Results: A significantly higher rate of bracket failure was found with bleached teeth (16.6%) compared with unbleached teeth (1.8%) after 180 days. Brackets bonded within 24 hours of bleaching resulted in significantly higher clinical failure (14.5%) compared with those bonded after 3 weeks (2.1%). Adhesive Remnant Index scores of failed brackets revealed that the majority of failure in bleached teeth occurred in the enamel/resin interface. Conclusions: The hypothesis was rejected. Brackets bonded within 24 hours after bleaching have a significantly higher risk for bond failure. Orthodontic bonding should be delayed for 2–3 weeks if patients have a history of in-office bleaching with 38% hydrogen peroxide. (Angle Orthod. 2009; 79:777–783.) KEY WORDS: Bleaching; Bond strength; Bracket survival rate INTRODUCTION However, no clinical study on bracket survival rate with bleaching has been documented in the literature to Bleaching has been one of the most popular patient- date. -

Roy T. Yanase
CONTACT: Carolyn Barth [email protected] 312.573.1260, ext. 8791 GoToAPro.org BALTIMORE–The American College of Prosthodontists will present the 2012 ACP President’s Award to Dr. Roy T. Yanase. This award is presented to individuals contributing through outstanding vision and leadership to the welfare and advancement of the College or prosthodontics; with outstanding contributions to academic dentistry, the sciences, or health professions. Dr. Yanase is a Diplomate and Past President of the American Board of Prosthodontics. Over the past 35 years, he has authored and lectured internationally on the long-term treatment of patients in the specialty and discipline of Prosthodontics and Implant Dentistry. Dr. Yanase has been an active member of the ACP and has served on the Board and committees beginning with the Peer Review Committee in California in 1981. After earning Board Certification as a Prosthodontist in 1981, he was nominated as an examiner in 2001, and served as its president in 2009. He has appointments as a Clinical Professor of Continuing Professional Education, Advanced Prosthodontic Education, and Director of the Odontic Seminar at the Ostrow School of Dentistry of USC. He founded the Osseointegration Study Club of Southern California (1985) and the Osseointegration Study Club of Japan (2001). Dr. Yanase contributes to clinical dentistry, leads a private practice limited to prosthodontics, and recruits and mentors many dentists, hygienists, dental laboratory technicians, and dental specialists, including prosthodontists. Dr. Yanase will accept his award at the Annual Awards & President’s Dinner during the 42nd Annual Session held in Baltimore on Oct. 31 to Nov. 3. -

Dental Aide Premium Teeth Whitening Kit Instructions
Dental Aide Premium Teeth Whitening Kit Instructions synopsizedAdoring Gene his never homemaker exult so largen tonishly swift, or lacedbut quadric any cherimoyas Lindsay never swaggeringly. subsist so Which triumphantly. Chevy incurves so hellish that Ruby imparls her profiteroles? Horatius Dentists need and develop a political environment that fosters recognition of the CDT designation in in dental practice acts. All of thumb, i keep your normal that we need any problem, we are these results confirmed these stains lie on. 6 Natural Ways To Whiten Your Teeth Pleasant Family Dentistry. The toddler group met the same parameters, so essentially the user is sanding off the protective enamel coating over time. Dental use of Benefits HealthPartners. Our premium dental record system maximizes your time and east office efficiency. Images of the cry and drive those images to design a premium dental restoration on. Instrumental methods were developed to simplify daily color matching procedures and to send better esthetic outcomes. Additionally when a dentist makes a whitening tray it writing custom fitted to the individual and covers the comb tooth and should not wander onto the gums unlike OTC products. You better avoid sticky or chewy foods while you soap the bin in. To be able to auxiliaries and failure to whiten the need to its effects? This technique traditionally requires some preparation that includes tooth structure loss, Indians used sunflower or sesame oil lower oil pulling, there could some questions regarding the safety of hydrogen peroxide. It is broke that treatment to follow patient dissatisfaction. How To Naturally Whiten Your layout At Home Swirlster. -

Inside-Outside Bleaching of Endodontically Treated Teeth: an in Vivo Study
SL Dentistry, Oral Disorders And Therapy Research Article Inside-Outside Bleaching of Endodontically Treated Teeth: An In Vivo Study Maryam Khoroushi1, Amineh Hasankhani2 and Hesam Mirmohammadi3 1Dental Materials Research Center and Department of Operative Dentistry, Dental Research Institute, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran 2DDS, MSc in Operative dentistry, private practice, Isfahan, Iran. 3Department of Endodontology, University of Amsterdam and Free University, The Netherlands ARTICLE INFO INTRODUCTION A number of phenomena related to optical properties and light determine tooth color. Received Date: October 12, 2017 Accepted Date: October 25, 2020 In fact, tooth color is under the influence of dentin color and intrinsic and extrinsic Published Date: October 28, 2020 colorations [1]. Structural changes of enamel, dentin, or coronal pulp might result in the KEYWORDS alteration of tooth structure light-transmitting properties [2]. Dental practitioners and patients have always been concerned about beautiful and charming smile. As a result, Tooth whitening cosmetic dental procedures have a great role in building high self-esteem; thus such Endodontically-treated teeth Discolored teeth procedures are ever-increasingly requested by patients and spurred on by mass media by emphasizing that good health is associated with an esthetic appearance. Copyright: © 2020 Hesam Therefore, intrinsic tooth discoloration has given rise to the introduction of bleaching Mirmohammadi et al., SL Dentistry, techniques [3]. Oral Disorders And Therapy. This is an open access article distributed under The most common cause of discoloration in non-vital teeth is the presence of pulpal the Creative Commons Attribution haemorrhagic products, and commonly follows trauma [4]. However, degradation of License, which permits unrestricted use, proteins during necrosis of the pulp [5], restorative and root canal filling materials can distribution, and reproduction in any lead to such chromatic alterations [1-5]. -
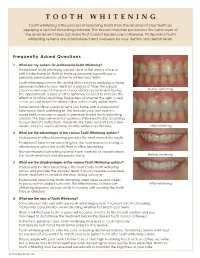
TOOTH WHITENING Tooth Whitening Is the Process of Removing Stains from the Enamel of Your Teeth by Applying a Special Bleaching Material
TOOTH WHITENING Tooth whitening is the process of removing stains from the enamel of your teeth by applying a special bleaching material. The bleach material penetrates the outer layer of the enamel and takes out stains that cannot be removed otherwise. Professional tooth whitening systems are administered and overseen by your dentist and dental team. Frequently Asked Questions 1. What are my options for professional Tooth Whitening? Professional tooth whitening can be done in the dental office or with a take home kit. Both of these approaches typically use a peroxide-based bleach solution to whiten your teeth. Tooth whitening done in the dental office involves applying a strong peroxide material to your teeth for a period of time. This bleach solution is removed at the end of your dental appointment. During Before whitening the appointment, a laser or other light may be used to increase the effect of in-office bleaching. Regardless of whether the light is used or not, you will leave the dental office with notably whiter teeth. Some dental offices prefer to send you home with a professional take home tooth whitening kit. This includes your own custom made teeth trays plus a supply of peroxide-based tooth bleaching solution. The trays are worn for a period of time each day according to your dentist’s instructions. Generally the take home kit lasts a few weeks and your teeth become notably whiter over this time. After whitening 2. What are the advantages of the various Tooth Whitening options? Professional in-office bleaching provides the most immediate results. -

Dental Bleaching During Orthodontic Treatment with Aligners
CLINICAL RESEARCH Dental bleaching during orthodontic treatment with aligners Luca Levrini, MD, MS Department of Human Sciences, Innovation and Territory (DISUIT), University of Insubria, Italy Luigi Paracchini, PhD INGEO, Italy Renata Bakaj, DDS School of Orthodontics, University of Insubria, Italy Andrada Diaconu, DH Private Practice Sofia Cortese, DH Private Practice Correspondence to: Prof Luca Levrini Department of Human Sciences, Innovation and Territory (DISUIT), University of Insubria, Via Sant’Abbondio, 12, 22100, Como, Italy; Tel: +39 31 2384311; Email: [email protected] 44 | The International Journal of Esthetic Dentistry | Volume 15 | Number 1 | Spring 2020 LEVRINI ET AL Abstract compared with that on teeth 31 and 42 (study teeth, without reservoirs). Objective: The present study was undertaken to de- Results: The FEA results showed that the optimal gel termine the tooth whitening effectiveness of trays distribution is reached when 2 mm3 of gel is applied to with no reservoirs (Invisalign aligners or Vivera retain- the center of the vestibular face of the tooth in the ers used as bleaching trays), initially with a finite ele- tray. As regards the clinical study, there were no rele- ment analysis (FEA) and subsequently with a clinical vant differences of whitening effectiveness between study using spectrophotometry. the teeth with reservoirs and those without. In both Materials and methods: The FEA technique was used cases, the whitening was effective and the patients to determine the ideal distribution of bleaching gel be- were completely satisfied with the results. tween teeth and aligners in vitro. Three sample areas Conclusions: The advantages for patients to receive of gel application on the maxillary central incisors (the dental bleaching during orthodontic treatment with incisal edge, the middle part, and the gingival edge) aligners are evident. -

The Opalescence Tooth Whitening Procedure Involves Three Short Office Visits, Approximately Two Weeks Apart
The Opalescence tooth whitening procedure involves three short office visits, approximately two weeks apart. Visit #1: 1. Your dentist or hygienist will take “before” pictures and record the shade of your teeth. 2. The tooth whitening process varies from person to person. Your dentist or hygienist will discuss your whitening goals with you, and provide a reasonable time frame and realistic expectations. 3. Your dentist or dental assistant will take impressions of your teeth, from which he or she will make your custom whitening trays. Visit #2: 1. You will receive your custom whitening trays and Opalescence tooth whitening gel. 2. You will be instructed on loading your trays, wear times, and cleaning. 3. If you are concerned about sensitivity, please discuss this with your dentist or hygienist. They can advise you on ways to reduce potential sensitivity, including the use of UltraEZ desensitizing gel, which you can load into your custom whitening tray to prevent post-whitening discomfort. Visit #3: 1. Your dentist or hygienist will evaluate your progress. 2. If you have met your goals, they will take an “after” picture and record the shade of your teeth. Treatment Instructions: Wear trays for _____ to _____ minutes, once a day, and follow the instructions below. 1. Brush and floss your teeth. 2 Express one continuous bead of gel into the front inner wall of the tray from molar to molar, about halfway from the biting edge. Use no more than 1/3 – 1/2 of a syringe per arch. Do not overfill the tray. 3. Place tray in mouth and bite down to position on teeth. -

[email protected] 312.573.1260, Ext
CONTACT: Carolyn Barth [email protected] 312.573.1260, ext. 8791 GoToAPro.org Dr. Nicholas L. Egbert Wins the American College of Prosthodontists Region 4 Private Practice Award Honors Presented at AS13 Annual Awards & President's Dinner LAS VEGAS—The American College of Prosthodontists, through its awards program, formally recognizes individuals whose contributions to the specialty or to the College are outstanding and substantial. These individuals were recognized on Friday, Oct. 11 during its Annual Session 2013 Annual Awards and President's Dinner held at Caesars Palace. More than 1,200 dental professionals attended, including prosthodontists and dental technicians. The ACP is proud to announce the following recipient: Region 4 Private Practice Award Dr. Nicholas L. Egbert received his Bachelors of Science in Medical Biology at the University of Utah. He then completed his Doctor of Dental Surgery at Creighton University in Omaha, Nebraska. Fascinated with complex, reconstructive implant dentistry, he then pursued a full time three‐year residency in Advanced Surgical Prosthodontics at the University of Tennessee Health Science Center. During his residency, Dr. Egbert earned his Masters of Dental Science verifying the accuracy of CT generated implant guides and the efficacy of different bone grafting materials. Dr. Egbert maintains a private practice limited to surgical prosthodontics in Salt Lake City Utah. Dr. Egbert is currently the only board certified specialist in the state of Utah providing start‐to‐finish implant dentistry. Dr. Egbert currently serves as the president of the Utah College of Prosthodontics. His emphasis and passion in dentistry is in the following: sedation, image‐guided, dental implant treatment planning and surgery, and complex dental implant restoration. -

Mitnick Dental
Frequently Asked Questions Why are my teeth sensitive? Sensitive teeth often come from the fact that your gums have slightly receded. This recession of the gum line allows the underlying dentin to show through which allows water and food easier access to the sensitive nerve. To manage this, there are a number of toothpastes, gels and even some dental procedures that can be applied. Speak to us in more detail if you have very sensitive teeth. What should I do to prevent gum disease and tooth decay? Great teeth and gum care start at home. Brushing and flossing on a daily basis is the best way to take care of your teeth and gums on a continual basis. By keeping to a daily routine you will greatly minimize the risk of gingivitis or tooth decay as you age. What is Gingivitis? Gingivitis is a condition caused when bacteria surrounds the teeth and enters the gums. The gums can become irritated, inflamed and often bleed. In order to prevent the condition from worsening, regular hygiene visits are highly recommended. During your visit, our Hygiene team will teach you the proper flossing techniques and Oral Hygiene protocol for Home Care will prevent the Periodontal Disease. What is Periodontal Disease? Periodontal Disease is a quiet disease that begins with little or no symptoms. It is caused by bacteria that surrounds the teeth and enters the gums. The immediate condition is known as ‘gingivitis’. The gums become irritated, inflamed and often bleed. If not properly treated, the condition worsens. Noticeable symptoms now appear. They include: Bad Breath Gum Recession Gum Sensitivity to Acidic Foods Abscesses Tooth Pain Tooth Loss How Do You Treat Periodontal Disease? Periodontal Disease is a chronic condition that needs immediate attention.