Radioactive Educational Resources Marie Curie: Teaching Radioactive in the Classroom
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Martian Crater Morphology
ANALYSIS OF THE DEPTH-DIAMETER RELATIONSHIP OF MARTIAN CRATERS A Capstone Experience Thesis Presented by Jared Howenstine Completion Date: May 2006 Approved By: Professor M. Darby Dyar, Astronomy Professor Christopher Condit, Geology Professor Judith Young, Astronomy Abstract Title: Analysis of the Depth-Diameter Relationship of Martian Craters Author: Jared Howenstine, Astronomy Approved By: Judith Young, Astronomy Approved By: M. Darby Dyar, Astronomy Approved By: Christopher Condit, Geology CE Type: Departmental Honors Project Using a gridded version of maritan topography with the computer program Gridview, this project studied the depth-diameter relationship of martian impact craters. The work encompasses 361 profiles of impacts with diameters larger than 15 kilometers and is a continuation of work that was started at the Lunar and Planetary Institute in Houston, Texas under the guidance of Dr. Walter S. Keifer. Using the most ‘pristine,’ or deepest craters in the data a depth-diameter relationship was determined: d = 0.610D 0.327 , where d is the depth of the crater and D is the diameter of the crater, both in kilometers. This relationship can then be used to estimate the theoretical depth of any impact radius, and therefore can be used to estimate the pristine shape of the crater. With a depth-diameter ratio for a particular crater, the measured depth can then be compared to this theoretical value and an estimate of the amount of material within the crater, or fill, can then be calculated. The data includes 140 named impact craters, 3 basins, and 218 other impacts. The named data encompasses all named impact structures of greater than 100 kilometers in diameter. -

Kadiworking Paper Finalcorrected
ACADEMY OF EUROPEAN LAW EUI Working Papers AEL 2009/10 ACADEMY OF EUROPEAN LAW CHALLENGING THE EU COUNTER-TERRORISM MEASURES THROUGH THE COURTS edited by Marise Cremona, Francesco Francioni and Sara Poli EUROPEAN UNIVERSITY INSTITUTE , FLORENCE ACADEMY OF EUROPEAN LAW ROBERT SCHUMAN CENTRE FOR ADVANCED STUDIES Challenging the EU Counter-terrorism Measures through the Courts EDITED BY MARISE CREMONA , FRANCESCO FRANCIONI AND SARA POLI EUI W orking Paper AEL 2009/10 This text may be downloaded for personal research purposes only. Any additional reproduction for other purposes, whether in hard copy or electronically, requires the consent of the author(s), editor(s). If cited or quoted, reference should be made to the full name of the author(s), editor(s), the title, the working paper or other series, the year, and the publisher. The author(s)/editor(s) should inform the Academy of European Law if the paper is to be published elsewhere, and should also assume responsibility for any consequent obligation(s). ISSN 1831-4066 © 2009 Marise Cremona, Francesco Francioni and Sara Poli (editors) Printed in Italy European University Institute Badia Fiesolana I – 50014 San Domenico di Fiesole (FI) Italy www.eui.eu cadmus.eui.eu Abstract This collection of papers examines the implications of the European Court of Justice’s approach to UN-related counter-terrorism measures against individuals (so-called ‘smart sanctions’), as expressed by its ruling in Case C-402/05P Kadi v Council and Commission , in which it annulled an EC act implementing a UN Security Council resolution. The impact of this seminal judgment on the EC legal order, on its relationship with the UN Charter, and on the case-law of the European Court of Human rights is the theme of this collection. -

Source of Knowledge, Techniques and Skills That Go Into the Development of Technology, and Prac- Tical Applications
DOCUMENT RESUME ED 027 216 SE 006 288 By-Newell, Homer E. NASA's Space Science and Applications Program. National Aeronautics and Space Administration, Washington, D.C. Repor t No- EP -47. Pub Date 67 Note-206p.; A statement presented to the Committee on Aeronautical and Space Sciences, United States Senate, April 20, 1967. EDRS Price MF-$1.00 HC-$10.40 Descriptors-*Aerospace Technology, Astronomy, Biological Sciences, Earth Science, Engineering, Meteorology, Physical Sciences, Physics, *Scientific Enterprise, *Scientific Research Identifiers-National Aeronautics and Space Administration This booklet contains material .prepared by the National Aeronautic and Space AdMinistration (NASA) office of Space Science and Applications for presentation.to the United States Congress. It contains discussion of basic research, its valueas a source of knowledge, techniques and skillsthat go intothe development of technology, and ioractical applications. A series of appendixes permitsa deeper delving into specific aspects of. Space science. (GR) U.S. DEPARTMENT OF HEALTH, EDUCATION & WELFARE OFFICE OF EDUCATION THIS DOCUMENT HAS BEEN REPRODUCED EXACTLY AS RECEIVEDFROM THE PERSON OR ORGANIZATION ORIGINATING IT.POINTS OF VIEW OR OPINIONS STATED DO NOT NECESSARILY REPRESENT OFFICIAL OMCE OFEDUCATION POSITION OR POLICY. r.,; ' NATiONAL, AERONAUTICS AND SPACEADi4N7ISTRATION' , - NASNS SPACE SCIENCE AND APPLICATIONS PROGRAM .14 A Statement Presented to the Committee on Aeronautical and Space Sciences United States Senate April 20, 1967 BY HOMER E. NEWELL Associate Administrator for Space Science and Applications National Aeronautics and Space Administration Washington, D.C. 20546 +77.,M777,177,,, THE MATERIAL in this booklet is a re- print of a portion of that which was prepared by NASA's Office of Space Science and Ap- -olications for presentation to the Congress of the United States in the course of the fiscal year 1968 authorization process. -

GRAIL Gravity Observations of the Transition from Complex Crater to Peak-Ring Basin on the Moon: Implications for Crustal Structure and Impact Basin Formation
Icarus 292 (2017) 54–73 Contents lists available at ScienceDirect Icarus journal homepage: www.elsevier.com/locate/icarus GRAIL gravity observations of the transition from complex crater to peak-ring basin on the Moon: Implications for crustal structure and impact basin formation ∗ David M.H. Baker a,b, , James W. Head a, Roger J. Phillips c, Gregory A. Neumann b, Carver J. Bierson d, David E. Smith e, Maria T. Zuber e a Department of Geological Sciences, Brown University, Providence, RI 02912, USA b NASA Goddard Space Flight Center, Greenbelt, MD 20771, USA c Department of Earth and Planetary Sciences and McDonnell Center for the Space Sciences, Washington University, St. Louis, MO 63130, USA d Department of Earth and Planetary Sciences, University of California, Santa Cruz, CA 95064, USA e Department of Earth, Atmospheric and Planetary Sciences, MIT, Cambridge, MA 02139, USA a r t i c l e i n f o a b s t r a c t Article history: High-resolution gravity data from the Gravity Recovery and Interior Laboratory (GRAIL) mission provide Received 14 September 2016 the opportunity to analyze the detailed gravity and crustal structure of impact features in the morpho- Revised 1 March 2017 logical transition from complex craters to peak-ring basins on the Moon. We calculate average radial Accepted 21 March 2017 profiles of free-air anomalies and Bouguer anomalies for peak-ring basins, protobasins, and the largest Available online 22 March 2017 complex craters. Complex craters and protobasins have free-air anomalies that are positively correlated with surface topography, unlike the prominent lunar mascons (positive free-air anomalies in areas of low elevation) associated with large basins. -

Constraining the Evolutionary History of the Moon and the Inner Solar System: a Case for New Returned Lunar Samples
Constraining the Evolutionary History of the Moon and the Inner Solar System: A Case for New Returned Lunar Samples The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Tartèse, Romain, et al. "Constraining the Evolutionary History of the Moon and the Inner Solar System: A Case for New Returned Lunar Samples." Space Science Reviews, 215 (December 2019): 54. © 2019, The Author(s). As Published http://dx.doi.org/10.1007/s11214-019-0622-x Publisher Springer Science and Business Media LLC Version Final published version Citable link https://hdl.handle.net/1721.1/125329 Terms of Use Creative Commons Attribution 4.0 International license Detailed Terms https://creativecommons.org/licenses/by/4.0/ Space Sci Rev (2019) 215:54 https://doi.org/10.1007/s11214-019-0622-x Constraining the Evolutionary History of the Moon and the Inner Solar System: A Case for New Returned Lunar Samples Romain Tartèse1 · Mahesh Anand2,3 · Jérôme Gattacceca4 · Katherine H. Joy1 · James I. Mortimer2 · John F. Pernet-Fisher1 · Sara Russell3 · Joshua F. Snape5 · Benjamin P. Weiss6 Received: 23 August 2019 / Accepted: 25 November 2019 / Published online: 2 December 2019 © The Author(s) 2019 Abstract The Moon is the only planetary body other than the Earth for which samples have been collected in situ by humans and robotic missions and returned to Earth. Scien- tific investigations of the first lunar samples returned by the Apollo 11 astronauts 50 years ago transformed the way we think most planetary bodies form and evolve. Identification of anorthositic clasts in Apollo 11 samples led to the formulation of the magma ocean concept, and by extension the idea that the Moon experienced large-scale melting and differentiation. -

Understanding the Lunar Surface and Space-Moon Interactions Paul Lucey1, Randy L
Reviews in Mineralogy & Geochemistry Vol. 60, pp. 83-219, 2006 2 Copyright © Mineralogical Society of America Understanding the Lunar Surface and Space-Moon Interactions Paul Lucey1, Randy L. Korotev2, Jeffrey J. Gillis1, Larry A. Taylor3, David Lawrence4, Bruce A. Campbell5, Rick Elphic4, Bill Feldman4, Lon L. Hood6, Donald Hunten7, Michael Mendillo8, Sarah Noble9, James J. Papike10, Robert C. Reedy10, Stefanie Lawson11, Tom Prettyman4, Olivier Gasnault12, Sylvestre Maurice12 1University of Hawaii at Manoa, Honolulu, Hawaii, U.S.A. 2Washington University, St. Louis, Missouri, U.S.A. 3University of Tennessee, Knoxville, Tennessee, U.S.A. 4Los Alamos National Laboratory, Los Alamos, New Mexico, U.S.A. 5Smithsonian Institution, Washington D.C., U.S.A. 6Lunar and Planetary Laboratory, Univ. of Arizona, Tucson, Arizona, U.S.A. 7University of Arizona, Tucson, Arizona, U.S.A. 8Boston University, Cambridge, Massachusetts, U.S.A. 9Brown University, Providence, Rhode Island, U.S.A. 10University of New Mexico, Albuquerque, New Mexico, U.S.A. 11 Northrop Grumman, Van Nuys, California, U.S.A. 12Centre d’Etude Spatiale des Rayonnements, Toulouse, France Corresponding authors e-mail: Paul Lucey <[email protected]> Randy Korotev <[email protected]> 1. INTRODUCTION The surface of the Moon is a critical boundary that shapes our understanding of the Moon as a whole. All geologic mapping and remote sensing techniques utilize only the outermost portion of the Moon. Before leaving the Moon for study in our laboratories, all lunar samples that have been studied existed at or very near the surface. With the exception of the deeply probing geophysical techniques, our understanding of the interior of the Moon is derived from surficial, but not superficial, information, coupled with geologic reasoning. -

In Crater Lake, OR
Studies ofIi HydrothermaljThcitk iNi ProcessesVL in Crater Lake, OR Robert W. Collier, Jack Dymond and James McManus College of Oceanography Oregon State University Corvallis, OR 97331 In Collaboration With: H. Phinney, D. Mclntire, G. Larson, M. Buktenica Oregon State University R. Bacon, C.'.. H. Nelson, J. H.,. Barber, Jr.ii, U.S.G.S., Menlo Park D KarlTh[ U. Hawaii J. Lupton U.C. Santa Barbara M.'i:i Watwood, C. Dahm U. of New Mexico A. Soutar, R. Weiss U. C. San Diego C. G. Wheat U. of Hawaii Submitted to: TheT National'V' Park Service, PNW Region Seattle, WA May 31, 1991 Cooperative Agreement No. CA 9000-3-0003 Subagreement No. 7 CPSU. College of Forestry, OSUji PATTULLO STUDY COLLEGE OF OCEANIC AND ATMOSPHERIC SCIENCES OSU College of Oceanography Repott # 90- 7 Studies of Hydrothermal Processes in Crater Lake, OR Robert W. Collier, Jack Dymond and James McManus College of Oceanography Oregon State University Corvallis, OR 97331 In Collaboration With: H. Phinney, D. Mclntire, G. Larson, M. Buktenica Oregon State University R. Bacon, C. H. Nelson, J. H. Barber, Jr. U.S.G.S., Menlo Park Karl U. Hawaii J. Lupton U.C. Santa Barbara M. Watwood, C. Dahm U. of New Mexico A. Soutar, R. Weiss U. C. San Diego C. G. Wheat U. of Hawaii Submitted to: The National Park Service, PNW Region Seattle, WA May 31, 1991 Cooperative Agreement No. CA 9000-3-0003 Subagreement No. 7 CPSU, College of Forestry, OSU OSU College of Oceanography Report #90-7 Executive summary Significant Observations Measurements of temperature and salt content within the South Basin of Crater Lake show surprising variations over distances of a few meters. -

Fluvial Regimes, Morphometry and Age of Jezero Crater Paleolake Inlet Valleys and Their Exobiological Significance for the 2020
Fluvial Regimes, Morphometry and Age of Jezero Crater Paleolake Inlet Valleys and their Exobiological Significance for the 2020 Rover Mission Landing Site Nicolas Mangold, Gilles Dromart, Véronique Ansan, Francesco Salese, Maarten Kleinhans, Marion Massé, Cathy Quantin-Nataf, Kathryn Stack To cite this version: Nicolas Mangold, Gilles Dromart, Véronique Ansan, Francesco Salese, Maarten Kleinhans, et al.. Flu- vial Regimes, Morphometry and Age of Jezero Crater Paleolake Inlet Valleys and their Exobiological Significance for the 2020 Rover Mission Landing Site. Astrobiology, Mary Ann Liebert, 2020,20(8), pp.994-1013. 10.1089/ast.2019.2132. hal-02933200 HAL Id: hal-02933200 https://hal.archives-ouvertes.fr/hal-02933200 Submitted on 8 Sep 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. ACCEPTED paper in Astrobiology Fluvial Regimes, Morphometry and Age of Jezero Crater Paleolake Inlet Valleys and their Exobiological Significance for the 2020 Rover Mission Landing Site N. Mangold1, G. Dromart2, V. Ansan1, F. Salese3,4, M.G. Kleinhans3, M. Massé1, C. Quantin2, K.M. Stack5 1 Laboratoire Planétologie et Géodynamique, UMR6112 CNRS, Nantes Université, Université Angers, 44322 Nantes, France; 2 Laboratoire de Géologie de Lyon, ENSLyon, Université Claude Bernard, CNRS, Lyon, France; 3 Faculty of Geosciences, Utrecht University, Utrecht, The Netherlands; 4 International Research School of Planetary Sciences, Università Gabriele D’Annunzio, Pescara, Italy. -

The George Wright Forum
national park units A new method for setting preservation priorities Science vs. political interference in system planning What's a name worth? — The Yosemite trademark fight Why public financing is critical to parks Needed: Reliable funding for bison management Countering "ecomodernism" The George Wright Forum The GWS Journal of Parks, Protected Areas & Cultural Sites volume 33 number 1 • 2016 Mission The George Wright Society promotes protected area stewardship by bring ing practitioners together to share their expertise. Our Goal The Society strives to be the premier organization connecting people, plac es, knowledge, and ideas to foster excellence in natural and cultural resource management, research, protection, and interpretation in parks and equivalent reserves. Board of Directors Nathalie Gagnon, President • Ottawa, Ontario Jerry M. Mitchell, Vice President • Littleton, Colorado David J. Parsons, Secretary • Florence, Montana Ryan Sharp, Treasurer • Manhattan, Kansas Zarnaaz Bashir • Ashburn, Virginia David Graber • Three Rivers, California Barrett Kennedy • Baton Rouge, Louisiana Armando Quintero • San Rafael, California Chris Spence • Mill Valley, California Lynn Wilson • Cobble Hill, British Columbia Graduate Student Liaison to the Board Gina Depper • Clemson, South Carolina Executive Office David Harmon, Executive Director/ Co-editor, The George Wright Forum Emily Dekker-Fiala, Conference Coordinator Rebecca Conard, Co-editor, The George Wright Forum P. O. Box 65 • Hancock, Michigan 49930-0065 USA 1-906-487-9722 • [email protected] • www.georgewright.org O 2016 The George Wright Society. All rights reserved (No copyright is claimed for previously published material reprinted herein.) ISSN 0732-4715. Editorial and manuscript submission guidelines may be found at www.georgewright.org/forum. Text paper is made of 50% recycled fi bers. -
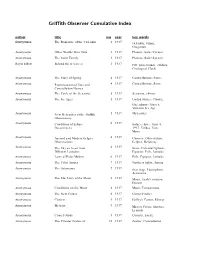
Griffith Observer Cumulative Index
Griffith Observer Cumulative Index author title mo year key words Anonymous The Romance of the Calendar 2 1937 calendar, Julian, Gregorian Anonymous Other Worlds than Ours 3 1937 Planets, Solar System Anonymous The S ola r Fa mily 3 1937 Planets, Solar System Roya l Elliott Behind the Sciences 3 1937 GO, pla ne ta rium, e xhibits , Ge ologica l Clock Anonymous The Stars of Spring 4 1937 Cons te lla tions , S ta rs , Anonymous Pronunciation of Star and 4 1937 Cons te lla tions , S ta rs Constellation Names Anonymous The Cycle of the Seasons 5 1937 Seasons, climate Anonymous The Ice Ages 5 1937 United States, Climate, Greenhouse Gases, Volcano, Ice Age Anonymous New Meteorites at the Griffith 5 1937 Meteorites Observatory Anonymous Conditions of Eclipse 6 1937 Solar eclipse, June 8, Occurrences 1937, Umbra, Sun, Moon Anonymous Ancient and Modern Eclipse 6 1937 Chinese, Observation, Observations Eclips e , Re la tivity Anonymous The Sky as Seen from 6 1937 Stars, Celestial Sphere, Different Latitudes Equator, Pole, Latitude Anonymous Laws of Polar Motion 6 1937 Pole, Equator, Latitude Anonymous The Polar Aurora 7 1937 Northern lights, Aurora Anonymous The Astrorama 7 1937 Star map, Planisphere, Astrorama Anonymous The Life Story of the Moon 8 1937 Moon, Earth's rotation, Darwin Anonymous Conditions on the Moon 8 1937 Moon, Temperature, Anonymous The New Comet 8 1937 Come t Fins le r Anonymous Comets 9 1937 Halley's Comet, Meteor Anonymous Meteors 9 1937 Meteor Crater, Shower, Leonids Anonymous Comet Orbits 9 1937 Comets, Encke Anonymous -

Université Pierre Et Marie Curie
Université Pierre et Marie Curie Ecole doctorale Complexité du Vivant Unité de Biologie des Interactions Cellulaires / URA CNRS 2582 The intracellular pathogen Chlamydia trachomatis targets proteins of the ESCRT machinery Par François VROMMAN Thèse de doctorat de Microbiologie Cellulaire Dirigée par Agathe SUBTIL Présentée et soutenue publiquement le 10 juin 2014 Devant un jury composé de : Pr. Vincent MARECHAL (Professeur UPMC) Président Dr. Reynaldo CARABEO (Directeur de laboratoire) Rapporteur Dr. Guy TRAN VAN NHIEU (Directeur de laboratoire) Rapporteur Dr. Nolwenn JOUVENET (Chargée de recherche) Examinatrice Dr. Agathe SUBTIL (Directrice de laboratoire) Directrice de thèse ACKNOWLEDGMENTS Je veux tout d’abord remercier la Pr. Alice Dautry qui a accepté de m’accueillir dans son laboratoire. Je la remercie pour ses remarques concernant mon travail malgré ses obligations de Directrice de l’Institut Pasteur, ainsi que pour ses conseils avisés notamment en communication. Je veux particulièrement remercier Agathe Subtil, ma directrice de thèse pour son implication dans ma thèse sur tous les points. Je suis reconnaissant de l’autonomie qu’elle a su me donner au cours de ces quatre années passées ici. Sa présence pour la préparation de communication et son travail de relecture, jusqu’à l’écriture de ce manuscrit, m’ont permis d’apprendre de mes erreurs et de progresser. Enfin je tiens à la remercier pour son soutien dans mes démarches extra-professionnelles (comme StaPa), dans le recrutement de stagiaires de l’université de Metz, ou encore pour les différentes conférences internationales auxquelles j’ai pu participer. Pour tout ça Agathe, MERCI. Merci à Stéphanie Perrinet pour son aide et son expertise technique, et surtout sa bonne humeur. -

Crater Lake Habitat Predicts Morphological Diversity in Adaptive Radiations of Cichlid Fishes
CRATER LAKE HABITAT PREDICTS MORPHOLOGICAL DIVERSITY IN ADAPTIVE RADIATIONS OF CICHLID FISHES 1 1 Hans Recknagel, •2 Kathryn R. Elmer, •2 and Axel Meyer1.3 1 Lehrstuhl far Zoologie und Evolutionsbiologie, Department of Biology, University of Konstanz, 78457 Konstanz, Germany 2/nstitute of Biodiversity, Animal Health and Comparative Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow G 12 BQQ, United Kingdom 3 E-mail: axel. meyer@uni-konstanz. de Adaptive radiations provide an excellent opportunity for studying the correlates and causes for the origin of biodiversity. In these radiations, species diversity may be influenced by either the ecological and physical environment. intrinsic lineage effects, or both. Disentangling the relative contributions of these factors in generating biodiversity remains a major challenge in understanding why a lineage does or does not radiate. Here, we examined morphological variation in body shape for replicate flocks of Nicaraguan Midas cichlid fishes and tested its association with biological and physical characteristics of their crater lakes. We found that variability of body elongation, an adaptive trait in freshwater fishes, is mainly predicted by average lake depth (N = 6, P < 0.001, R2 = 0.96). Other factors considered, including lake age, surface area, littoral zone area, number of co-occurring fish species, and genetic diversity of the Midas flock, did not significantly predict morphological variability. We also showed that lakes with a larger littoral zone have on average higher bodied Midas cichlid s, indicating that Midas cichlid flocks are locally adapted to their crater lake habitats. In conclusion, we found that a lake's habitat predicts the magnitude and the diversity of body elongation in repeated cichlid adaptive radiations.