L35 Official Journal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Składy Obwodowych Komisji Wyborczych W Gminie Izbica Po Dokonaniu Wyboru Przewodniczących Oraz Zastępców Komisji Obwodowa Komisja Wyborcza Nr 1 W Izbicy, Ul
Składy Obwodowych Komisji Wyborczych w Gminie Izbica po dokonaniu wyboru Przewodniczących oraz Zastępców Komisji Obwodowa Komisja Wyborcza Nr 1 w Izbicy, ul. Gminna 4, 22-375 Izbica Lp. Imię i nazwisko członka Miejsce Zgłoszona/y przez OKW zamieszkania 1. Aleksander Leon Wołos Tarnogóra KWW „Jerzego Lewczuka" Przewodniczący Komisji 2. Zygmunt Jagoda - Zastępca Izbica KW Prawo i Sprawiedliwość Przewodniczącego Komisji 3. Marta Małgorzata Ciastoch Tarnogóra Komitet Wyborczy PSL 4. Ewa Danielak Izbica KW Samoobrona 5. Iwona Nowosadzka Tarzymiechy Drugie KWW Łukasza Bartnika 6. Monika Olech Izbica KWW Justyny Popis 7. Jarosław Lisowski Izbica KWW Ruch Narodowy 8. Gabriel Mazurek Wirkowice Pierwsze KW Twój Ruch 9. Czesław Swistowski Izbica pracownik samorządowy 11 11 — — Obwodowa Komisja Wyborcza Nr 2 w Wólce Orłowskiej 133 Lp. linię i nazwisk;V; o członka Adres zamieszkania Zgłoszona/y przez ^HHM ''' i H! :i i:! i:; i!:!: ;;; l!|il|i!i!lftBl|:Ill • OKW ^•łll^^JlllliWF : 1. Jan Stanisław Bereza - Wólka Orłowska KWW Karola Jerzego Babiarza Przewodniczący Komisji 2. Maria Tałanda - Zastępca Izbica KW Platforma Obywatelska RP Przewodniczącego Komisji 3. Elżbieta Krystyna Bednarz Wólka Orłowska Komitet Wyborczy PSL 4. Wioletta Bartyka Wólka Orłowska KWW „Jerzego Lewczuka" 5. Anna Dorota Nizioł Wólka Orłowska KW Samoobrona 6. Henryk Roman Brzozowski Kryniczki KW Prawo i Sprawiedliwość 7. Anna Kryłowicz Izbica KWW Ruch Narodowy 8. Bogdan Michał Potapiński Kol.Orłów Murowany KW Twój Ruch 9. Mirosława Kalisz Izbica pracownik samorządowy Obwodowa Komisja Wyborcza Nr 3 w Orłowie Drewnianym 60A Lp. Imię i nazwisko członka Adres zamieszkania Zgłoszona/y przez OK W f: - ' - :- - . - : 1. Renata Paradowska - Izbica KWW Ruch Narodowy Przewodniczący Komisji 2. -

Harmonogram Odbioru Odpadów- GMINA MAŁDYTY
2019 HARMONOGRAM ODBIORU ODPADÓW KOMUNALNYCH GMINA MAŁDYTY 1 niesegregowane(zmieszane) odpady komunalne MIEJSCOWOŚĆ styczeń luty marzec kwiecień maj czerwiec lipiec sierpień wrzesień październik listopad grudzień 2,16,30, 13,27 13,27 10,24 8,22 5,19 3,17,31 14,28 11,25 9,23 6,20, 4,18 POJAZD NR 1 Zbiórka selektywna w workach (PLASTIK) KARCZEMKA styczeń luty marzec kwiecień maj czerwiec lipiec sierpień wrzesień październik listopad grudzień RYBAKI GUMISKA 11 8 8 12,26 10,24 7,21 5,19 2,16,30, 13,27 11,25 8 6 WIELKIE GUMISKA MAŁE Zbiórka selektywna w workach(SZKŁO) BUDWITY BUDYTY styczeń luty marzec kwiecień maj czerwiec lipiec sierpień wrzesień październik listopad grudzień GIZAJNY 14 11 11 8 6 10 8 5 9 7 4 9 KADZIE KREKI Zbiórka selektywna w workach(MAKULATURA) SASINY NIEDŹWIADA styczeń luty marzec kwiecień maj czerwiec lipiec sierpień wrzesień październik listopad grudzień POŁOWITE KLONOWY DWÓR 11 8 8 12 10 7 5 2 13 11 8 6 KANTY odpady zielone oraz odpady komunalne ulegające biodegradacji POJAZD NR 2 styczeń luty marzec kwiecień maj czerwiec lipiec sierpień wrzesień październik listopad grudzień DZIŚNITY 14 11 11 8,23 6,20, 3,17 1,15,29 12,26 9,23 7,21 4 9 SOPLE LINKI popiół LESZCZYNKA PLEŚNO styczeń luty marzec kwiecień maj czerwiec lipiec sierpień wrzesień październik listopad grudzień BAGNITY SURZYKI WIELKIE 14,28 11,25 11,25 8,23 6 10 8 5 9 7,21 4,18 2,16,30 SURZYKI MAŁE SZYMONOWO zużyty sprzęt elektryczny i elektroniczny SZYMONÓWKO 21 marca 2019 5 czerwca 2019 19 września 2019 11 grudnia 2019 PLĘKITY WODZIANY meble i inne odpady wielkogabarytowe 21 marca 2019 5 czerwca 2019 19 września 2019 11 grudnia 2019 Odbiór z nieruchomości niezamieszkałych z zadeklarowaną częstotliwością inną niż dwa razy w miesiącu odbywał się będzie na podstawie indywidualnych ustaleń. -

Program Rozwoju Lokalnego Powiatu Ostródzkiego
PROGRAM ROZWOJU LOKALNEGO POWIATU OSTRÓDZKIEGO Ostróda, lipiec 2004 r. Spis Treści ........................................................................................................................ 1 Wstęp......................................................................................................................................... 4 I. Obszar i czas realizacji Programu Rozwoju Lokalnego...................................................... 6 II. Aktualna sytuacja społeczno-gospodarcza na obszarze planowania.................................... 7 1. Położenie, powierzchnia, ludność ................................................................................... 7 2. Środowisko przyrodnicze.............................................................................................. 10 2.1. Charakterystyka fizjograficzna powiatu................................................................. 10 2.2. Gleby .................................................................................................................... 10 2.3. Kopaliny ............................................................................................................... 11 2.4. Sieć hydrograficzna............................................................................................... 12 2.5. Wody powierzchniowe.......................................................................................... 15 2.6. Wody gruntowe..................................................................................................... 16 2.7. Powietrze............................................................................................................. -

Uchwala Nr XXIV/139/2017 Z Dnia 30 Marca 2017 R
DZIENNIK URZĘDOWY WOJEWÓDZTWA LUBELSKIEGO Lublin, dnia 7 kwietnia 2017 r. Poz. 1690 UCHWAŁA NR XXIV/139/2017 RADY GMINY GORZKÓW z dnia 30 marca 2017 r. w sprawie dostosowania sieci szkoły podstawowej i gimnazjum do nowego ustroju szkolnego, wprowadzonego ustawą - Prawo oświatowe Na podstawie art. 18 ust. 2 pkt 15 ustawy z dnia 8 marca 1990 r. o samorządzie gminnym (Dz. U. z 2016 r. poz. 446, z późn. zm.) oraz art. 210 ustawy z dnia 14 grudnia 2016 r.- Przepisy wprowadzające ustawę - Prawo oświatowe (Dz. U. z 2017 r. poz. 60) po uzyskaniu pozytywnej opinii Lubelskiego Kuratora Oświaty w Lublinie, uchwala się, co następuje: § 1. Uchwała określa: 1) plan sieci publicznej szkoły podstawowej prowadzonej przez Gminę Gorzków, a także granice obwodu publicznej szkoły podstawowej prowadzonej przez Gminę Gorzków, na okres od 1 września 2017 r. do dnia 31 sierpnia 2019 r., który stanowi załącznik 1 do niniejszej uchwały; 2) plan sieci prowadzonych przez Gminę Gorzków klas dotychczasowego publicznego gimnazjum prowadzonego w szkole podstawowej oraz granice obwodu klas dotychczasowego publicznego gimnazjum prowadzonego przez Gminę Gorzków na okres od 1 września 2017 r. do dnia 31 sierpnia 2019 r., który stanowi załącznik 2 do niniejszej uchwały. § 2. Gimnazjum w Gorzkowie z siedzibą w Gorzkowie przy ul. Partyzantów 83, włącza się do Szkoły Podstawowej w Gorzkowie z siedzibą w Gorzkowie przy ul. Głównej 7 na następujących warunkach: 1) Szkoła Podstawowa w Gorzkowie rozpocznie działalność z dniem 1 września 2017 r.; 2) kształcenie w klasie I Szkoły Podstawowej w Gorzkowie rozpocznie się w roku szkolnym 2017/2018; 3) Gimnazjum w Gorzkowie zakończy działalność z dniem 31 sierpnia 2017 r. -

GMINA MAŁDYTY 1 Niesegregowane(Zmieszane) Odpady Komunalne
2020 HARMONOGRAM ODBIORU ODPADÓW KOMUNALNYCH GMINA MAŁDYTY 1 niesegregowane(zmieszane) odpady komunalne MIEJSCOWOŚĆ styczeń luty marzec kwiecień maj 3,15,29 12,26 11,25 8,22 6,20 KARCZEMKA Zbiórka selektywna w workach (PLASTIK) RYBAKI styczeń luty marzec kwiecień maj GUMISKA WIELKIE GUMISKA MAŁE 21 18 17 8,22 5,19 BUDWITY BUDYTY Zbiórka selektywna w workach(SZKŁO) GIZAJNY styczeń luty marzec kwiecień maj KADZIE KREKI 22 19 18 23 20 SASINY Zbiórka selektywna w workach(MAKULATURA) NIEDŹWIADA POŁOWITE styczeń luty marzec kwiecień maj KLONOWY DWÓR KANTY 21 18 17 8,22 5,19 SMOLNO odpady zielone oraz odpady komunalne ulegające biodegradacji DZIŚNITY styczeń luty marzec kwiecień maj SOPLE 23 20 19 9,23 7,21 LINKI LESZCZYNKA popiół BAGNITY SURZYKI WIELKIE styczeń luty marzec kwiecień maj SURZYKI MAŁE 8,23 6,2 4,19 9,23 21 SZYMONOWO SZYMONÓWKO zużyty sprzęt elektryczny i elektroniczny PLĘKITY 10 marca 2020 WODZIANY meble i inne odpady wielkogabarytowe 10 marca 2020 Odbiór z nieruchomości niezamieszkałych z zadeklarowaną częstotliwością inną niż dwa razy w miesiącu odbywać będzie się na podstawie indywidualnych ustaleń. W przypadku zadeklarowanego odbioru raz w m-cu odbiór z nieruchomości niezamieszkałych odbywać się będzie w drugim terminie miesiąca. Właściciele nieruchomości obowiązani są udostępnić odpady przed posesją do godziny 6:00 w dniu odbioru. 2020 HARMONOGRAM ODBIORU ODPADÓW KOMUNALNYCH GMINA MAŁDYTY 2 niesegregowane(zmieszane) odpady komunalne MIEJSCOWOŚĆ styczeń luty marzec kwiecień maj 4,17,31 14,28 13,27 10,24 8,22 Zbiórka selektywna -

W NUMERZE M.In.: Pomnika Powstańca Wielkopolskiego Przemówienie Burmistrza Wolsztyna Andrzeja Rogozinskiego Sesja Rady Miejskiej …….…
ISSN 2300-8652 02 (2014) SOŁECTWA: ADAMOWO, BARŁOŻNIA GOŚCIESZYŃSKA, BERZYNA, BŁOCKO, CHORZEMIN, GOŚCIESZYN, KARPICKO, KĘBŁOWO, NIAŁEK WIELKI, NOWA DĄBROWA, OBRA, NOWA OBRA, NOWE TŁOKI, NOWY WIDZIM, POWODOWO, RUDNO, STARY WIDZIM, STRADYŃ, STARA DĄBROWA, ŚWIĘTNO, TŁOKI, WILCZE, WRONIAWY Uroczyste odsłonięcie W NUMERZE m.in.: Pomnika Powstańca Wielkopolskiego Przemówienie Burmistrza Wolsztyna Andrzeja Rogozinskiego Sesja Rady Miejskiej …….…. s. 2 Drodzy Mieszkańcy naszego miasta ! Odsłonięcie Pomnika ….…... s. 4 Powstanie Wielkopolskie, jak słusznie podkreśla się – było jedynym w pełni udanym zrywem niepodle- Z wizytą w Błocku ………...... s. 6 głościowym Polaków. Jego tradycja na trwałe wpisała się w pamięć mieszkańców Wielkopolski. Nasi przodkowie, bez jakiejkolwiek pomocy zewnętrznej, chwyciwszy za broń przynieśli wolność swojej ziemi. OSP w Kębłowie ….….…….… s. 9 Walki uliczne rozpoczęte 27 grudnia 1918 r. w Poznaniu dały sygnał do rozpoczęcia powstania na terenie Poznańskiego, w szybkim tempie obejmując całą Wielkopolskę. Powstańcy zbrojnie odbijali z rąk zaborcy wioski i miasta, prowadząc na wielu odcinkach frontu niezwykle zacięte i krwawe walki. W ich wyniku 5 stycznia 1919 r. Biblioteka Miejska …….….. s. 10 wyzwolone zostało nasze miasto. Dokładnie w 95 rocznicę tego wydarzenia odbyła się uroczystość wmurowania aktu erekcyjnego pod Pomnik Powstańca Wielkopolskiego. Dziś zgromadziliśmy się kontynuując tamtą uroczy- stość. Wybór dzisiejszego dnia nie jest przypadkowy. 95 lat temu, 16 lutego, w Trewirze, podpisano rozejm for- malnie kończący powstanie wielkopolskie. Traktat ten był sukcesem politycznym Wielkopolan. Należy jednak pamiętać, że nie byłby możliwy, gdyby nie działania, jakie podjęli powstańcy. Rozejm w Trewirze poprzedziły pra- wie 2 miesiące walk. Gdyby nie sukcesy odnoszone przez powstańców, zapisy pokojowe nie byłyby dla strony polskiej tak korzystne. Powstanie Wielkopolskie 1918/1919 przyczyniło się do zmiany układu zachodniej granicy, gdyby nie wybuchło – status quo większości terenów Wielkopolski byłby z pewnością zupełnie inny. -

L5 Amtsblatt
Amtsblatt L 5 der Europäischen Union 63. Jahrgang Ausgabe in deutscher Sprache Rechtsvorschriften 9. Januar 2020 Inhalt II Rechtsakte ohne Gesetzescharakter BESCHLÜSSE ★ Durchführungsbeschluss (EU) 2020/10 der Kommission vom 7. Januar 2020 betreffend bestimmte vorläufige Maßnahmen zum Schutz vor der hochpathogenen Aviären Influenza des Subtyps H5N8 in Polen (Bekannt gegeben unter Aktenzeichen C(2020) 75) (1) . 1 (1) Text von Bedeutung für den EWR. Bei Rechtsakten, deren Titel in magerer Schrift gedruckt sind, handelt es sich um Rechtsakte der laufenden Verwaltung im Bereich der Agrarpolitik, die normalerweise nur eine begrenzte Geltungsdauer haben. DE Rechtsakte, deren Titel in fetter Schrift gedruckt sind und denen ein Sternchen vorangestellt ist, sind sonstige Rechtsakte. 9.1.2020 DE Amtsblatt der Europäischen Uni on L 5/1 II (Rechtsakte ohne Gesetzescharakter) BESCHLÜSSE DURCHFÜHRUNGSBESCHLUSS (EU) 2020/10 DER KOMMISSION vom 7. Januar 2020 betreffend bestimmte vorläufige Maßnahmen zum Schutz vor der hochpathogenen Aviären Influenza des Subtyps H5N8 in Polen (Bekannt gegeben unter Aktenzeichen C(2020) 75) (Nur der polnische Text ist verbindlich) (Text von Bedeutung für den EWR) DIE EUROPÄISCHE KOMMISSION — gestützt auf den Vertrag über die Arbeitsweise der Europäischen Union, gestützt auf die Richtlinie 89/662/EWG des Rates vom 11. Dezember 1989 zur Regelung der veterinärrechtlichen Kontrollen im innergemeinschaftlichen Handel im Hinblick auf den gemeinsamen Binnenmarkt (1), insbesondere auf Artikel 9 Absatz 3, gestützt auf die Richtlinie -

(EU) 2020/281 of 27 February 2020 Amending the Annex To
28.2.2020 EN Offi cial Jour nal of the European Union L 59/13 COMMISSION IMPLEMENTING DECISION (EU) 2020/281 of 27 February 2020 amending the Annex to Implementing Decision (EU) 2020/47 on protective measures in relation to highly pathogenic avian influenza of subtype H5N8 in certain Member States (notified under document C(2020) 1248) (Text with EEA relevance) THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union, Having regard to Council Directive 89/662/EEC of 11 December 1989 concerning veterinary checks in intra-Community trade with a view to the completion of the internal market (1), and in particular Article 9(4) thereof, Having regard to Council Directive 90/425/EEC of 26 June 1990 concerning veterinary checks applicable in intra-Union trade in certain live animals and products with a view to the completion of the internal market (2), and in particular Article 10(4) thereof, Whereas: (1) Commission Implementing Decision (EU) 2020/47 (3) was adopted following outbreaks of highly pathogenic avian influenza of subtype H5N8 in holdings where poultry are kept in certain Member States, and the establishment of protection and surveillance zones by those Member States in accordance with Council Directive 2005/94/EC (4). (2) Implementing Decision (EU) 2020/47 provides that the protection and surveillance zones established by the Member States listed in the Annex to that Implementing Decision, in accordance with Directive 2005/94/EC, are to comprise at least the areas listed as protection and surveillance zones in that Annex. (3) The Annex to Implementing Decision 2020/47 was recently amended by Commission Implementing Decision (EU) 2020/240 (5), following outbreaks of highly pathogenic avian influenza of subtype H5N8 in poultry in Bulgaria and Czechia that needed to be reflected in that Annex. -

Uchwała Nr XXXIII/274/2013 Z Dnia 30 Października 2013 R
DZIENNIK URZĘDOWY WOJEWÓDZTWA WIELKOPOLSKIEGO Poznań, dnia 4 listopada 2013 r. Poz. 5950 UCHWAŁA NR XXXIII/274/2013 RADY MIEJSKIEJ W WOLSZTYNIE z dnia 30 października 2013 r. w sprawie: opłaty od posiadania psów. Na podstawie art. 18 ust. 2 pkt 8, art. 40 ust. 1, art. 41 ust. 1 ustawy z dnia 8 marca 1990 roku o samorządzie gminnym (tekst jednolity z 2013 roku Dz. U. poz.594 z późniejszymi zmianami), art. 18a i 19 pkt 1 lit. f, pkt 2 i pkt 3 ustawy z dnia 12 stycznia 1991 roku o podatkach i opłatach lokalnych (tekst jednolity z 2010 roku Dz. U. Nr 95, poz. 613 z późniejszymi zmianami) uchwala się, co następuje: § 1. Uchwałą niniejszą Rady Miejskiej w Wolsztynie: 1) wprowadza się na terenie gminy opłatę od posiadania psów, 2) określa się wysokość stawki opłaty od posiadania psów, 3) określa się zasady ustalania i poboru oraz terminy płatności opłaty od posiadania psów, 4) zarządza się pobór opłaty od posiadania psów w drodze inkasa, określa inkasentów oraz wynagrodzenie za inkaso, 5) wprowadza się inne niż wymienione w ustawie zwolnienia przedmiotowe z opłaty od posiadania psów. § 2. Wprowadza się na terenie Gminy Wolsztyn opłatę od posiadania psów. § 3. Stawkę roczną opłaty od posiadania psów ustala się w wysokości 46,00 zł od jednego psa. § 4. Określa się następujące zasady ustalania opłaty od posiadania psów: 1) opłata od posiadania psów płatna jest bez wezwania do dnia 31 marca roku podatkowego, a przypadku nabycia psa po upływie terminu płatności w terminie 14 dni od daty zaistnienia okoliczności powodujących powstanie obowiązku uiszczenia opłaty. -

Wieści Z Gminy Ostroróg ISSN 2450-6060
Bezpłatny Biuletyn Informacyjny Gminy Ostroróg nr 2 (3) luty/marzec 2016 r. Wieści z Gminy Ostroróg ISSN 2450-6060 Zwycięski stół w pierwszym w Naszej Gminie Pokazie Stołów Wielkanocnych Program Policja prze- 65-lecie Wykaz wy- Fotorelacja „Rodzina prowadziła Koła PZW branych z gminnej 500+”. konsultacje nr 35 zadań zre- spartakiady Kto społeczne Ostroróg alizowanych zimowej skorzysta? w Ostrorogu w ubiegłym w Ostrorogu roku 2 | Bezpłatny Biuletyn Informacyjny Gminy Ostroróg nr 2 (3) luty/marzec 2016 r. SZANOWNI MIESZKAŃCY W lutym, po blisko dwóch mie- siącach przerwy odbyła się kolejna sesja Rady Miejskiej w Ostrorogu. W obradach uczestniczył Poseł na Sejm RP, Marcin Porzucek. W swo- im sprawozdaniu z działalności mię- dzysesyjnej sporo czasu poświęciłem ambitnemu i zakrojonemu na bar- dzo szeroką skalę programowi „Ro- dzina 500+”. W związku z licznymi pytaniami kierowanymi do naszych urzędników postanowiliśmy wyja- śnić niektóre kwestie z nim związa- ne. Dodam, że po konsultacjach zde- cydowaliśmy, że do obsługi programu w naszej gminie nie zatrudnimy no- sterstwa Sprawiedliwości. lepsze warunki dla rozwijania ta- wego pracownika. W tym numerze „Wieści z Gmi- lentów naszych sportowców i rekre- Na ostatniej sesji Rady Miejskiej ny Ostroróg” chcemy przybliżyć acji pozostałych osób zamieszkują- w urzędzie gościliśmy nie tylko par- Wam zadania zrealizowane w ubie- cych Gminę. lamentarzystę, ale także Starostę Sza- głym roku. Plan inwestycyjny wyni- Zapewne niewiele osób wie, jaką motulskiego Józefa Kwaśniewicza, kający z podjętej przez Radę Miejską historię kryją w sobie miejscowo- członka zarządu powiatu Andrze- w Ostrorogu uchwały budżetowej ści znajdujące się na terenie Naszej ja Grzeszczyka oraz dwóch radnych został wykonany. Cieszę się, że nowy Gminy. -
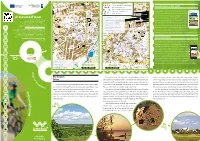
Web Portal Z P R R P Wojennych Ie I Z BRZESKA M R R N S a Ł R H O Z R Żo EJOW LUBLIN B O IECK Palaces and Mansions; Museums P M E A
a g u ł D B hemiczna i C iego e lsk W Ch Po ł emiczn wa ojska a a o y Targ W w g i n o zafirowa n ów S irak yb A S C . a k F AL. PRZYJAŹN h o I a r s ł East of Poland Cycling Trail Green Velo e a y a TERESPOL g i c s w m z E373 z 12 u y c 816 n o ł a n i t K r c i I DOROHUSK z y - m a D o N s z A M e Churches; cloisters; Orthodox churches;w synagogues Ż Ź d na s k n N cz L a z a A Cmentarz Jeńców i a n o t ł a W o ar z oc r J T . łn O y ó d a P e Y www.greenvelo.pl web portal z p R r P Wojennych ie i Z BRZESKA M r r n s A ł R H o z R Żo EJOW LUBLIN B o IECK Palaces and mansions; museums P M e A A A K w . L S w U . ZEA P BRSK G 82 B E R L K 12 PA Z a EL E373 A A RAPMA BR s S REJ M S o KA OW RA Żytnia a IECK k A A a Z SK ł g L ł E i Hotels; tourist information centres; parking facilities B n e ą z A . U K u L ES Z ł a R t d g B iN c PA J a AM W o R o m k D R zy KA . -

Wykaz Kół Łowieckich Na Terenie Powiatu Ostrów
Wykaz kół łowieckich na terenie powiatu Ostrów numer miejscowości przynależne do powiat gmina koło łowieckie adres korespondencyjny mail Prezes Koła obwodu obwodu łowieckiego Ostrów Wielkopolski (m.) Sławin, Ołobok, Rososzyca, Sieroszewice, Parczew, Bibianki, Biskupice Ołoboczne, K.Ł. Nr 7 "BAŻANT" Ostrów Skalmierzyce, Bilczew, Psary, Moszczanka 118, 63-440 Raszków 453 [email protected] Jacek Kopecki Wlkp. Gostyczyna, Chotów, Węgry, Mączniki, Śmiłów, Osiek, Żydów, Strzegowa, Śliwniki, Sulisławice Czekanów, Młynów, Biskupice, Kościuszków, Wtórek, Czachory, Parczew, Sieroszewice, Trąba, Ostrów W.K.Ł. Nr 49 "SZARAK" Ostrów Leśnictwo Wtórek, Kąkolewo 1, 63- 452 Wlkp., Gniazdów, Fabianów, [email protected] Andrzej Perz Wlkp. 400 Ostrów Wlkp. Kęszyce, Nowe Skalmierzyce, Boczków, Trusków, Kunów, Nowe Skalmierzyce Zaborów, Miedzianów, Gałązki Małe, Kawetczyzna, Kwiatków Borowiec, Sobótka, Gutów, Gałązki Małe, Gałązki Wielkie, Droszew, Kotowiecko, K.Ł. Nr 10 "ZALESIE" Ostrów Wisławka 1, 63-430 Odolanów 449 Miedzianów, Trkusów, Głóski, [email protected] Jan Mróz Wlkp. Kościelna Wieś, Czechel, Karsy, Żychlin, Borucin, Grudzielec, Nowa Wieś Biskupice, Boczków, Chotów, Głóski, Gniazdów, Kalisz, ul. Stefana Batorego 1/14, 63-300 W.K.Ł. Nr 47 "ORĘŻ" Pleszew 391 Szczypiorno, Kościelna Wieś, [email protected] Andrzej Biesiada Pleszew Murowaniec, Skalmierzyce, Sulisławice, Trkusów, Żydów Drogosław, Walentynów, Janków Zaleśny, Pogrzybów, Przybysławice, Raszków, K.Ł. Nr 10 "ZALESIE" Ostrów Niemojewiec, Sulisław, Wisławka 1, 63-430 Odolanów 450 [email protected] Jan Mróz Wlkp. Wierzbno, Tarchały Małe, Gorzyce Małe, Radziwiłów, Gorzyce Wielkie, Lamki, Zalesie, Świeligów, Jaskółki Biadki, Janków, Zaleśny, Łąkociny, Doniszyn, Wierzbno, Gliśnica, Odolanów, Raczyce, ul. Narutowicza 4, 63-400 Ostrów Sulmierzyce, Chwaliszew, K.Ł. Nr 43 "PONOWA" Biadki 454 Jan Pabich Wlkp. Świnków, Warszty, Mazury, Cegły, Chruszczyny, Nabyszyce, Kuroch, Kaczory, Uciechów Odolanów Dębnica, Czarny Las, Ludwików, Antonin, Kozły, Antonin, ul.