Is Morphology Really at Odds with Molecules in Estimating Fern Phylogeny?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Origin and Early Evolution of Plants on Land
review article The origin and early evolution of plants on land Paul Kenrick & Peter R. Crane . The origin and early evolution of land plants in the mid-Palaeozoic era, between about 480 and 360 million years ago, was an important event in the history of life, with far-reaching consequences for the evolution of terrestrial organisms and global environments. A recent surge of interest, catalysed by palaeobotanical discoveries and advances in the systematics of living plants, provides a revised perspective on the evolution of early land plants and suggests new directions for future research. The origin and early diversification of land plants marks an interval Eoembryophytic (mid-Ordovician [early Llanvirn: ϳ476 Myr] to of unparalleled innovation in the history of plant life. From a simple Early Silurian [late Llandovery: ϳ432 Myr])3. Spore tetrads (com- plant body consisting of only a few cells, land plants (liverworts, prising four membrane-bound spores; Fig. 2d) appear over a broad hornworts, mosses and vascular plants) evolved an elaborate two- geographic area in the mid-Ordovician and provide the first good phase life cycle and an extraordinary array of complex organs and evidence of land plants3,26,29. The combination of a decay-resistant tissue systems. Specialized sexual organs (gametangia), stems with wall (implying the presence of sporopollenin) and tetrahedral an intricate fluid transport mechanism (vascular tissue), structural configuration (implying haploid meiotic products) is diagnostic tissues (such as wood), epidermal structures for respiratory gas of land plants. The precise relationships of the spore producers exchange (stomates), leaves and roots of various kinds, diverse within land plants are controversial, but evidence of tetrads and spore-bearing organs (sporangia), seeds and the tree habit had all other spore types (such as dyads) in Late Silurian and Devonian evolved by the end of the Devonian period. -

A Revision of Schoenobryum (Cryphaeaceae, Bryopsida) in Africa1
Revision of Schoenobryum 147 Tropical Bryology 24: 147-159, 2003 A revision of Schoenobryum (Cryphaeaceae, Bryopsida) in Africa1 Brian J. O’Shea 141 Fawnbrake Avenue, London SE24 0BG, U.K. Abstract. The nine species and two varieties of Schoenobryum reported for Africa were investigated, and no characters were found that uniquely identified any of the taxa to be other than the pantropical Schoenobryum concavifolium. The following nine names become new synonyms of S. concavifolium: Cryphaea madagassa, C. subintegra, Acrocryphaea robusta, A. latifolia, A. subrobusta, A. tisserantii, A. latifolia var. microspora, A. plicatula and A. subintegra var. idanreense; a lectotype is selected for Acrocryphaea latifolia var. microspora P.de la Varde. INTRODUCTION as the majority have not been examined since the type description, and many have never been A recent checklist of Sub-Saharan Africa illustrated. (O’Shea, 1999) included nine species and two varieties of Schoenobryum, most of quite limited The purpose of this paper is to provide an distribution. Recent collecting in both Malawi overview of the genus worldwide, and to review (O’Shea et al., 2001) and Uganda (Wigginton et the taxonomic position of the African taxa. al., 2001) has shown the genus to be not uncommon, although there was only one CRYPHAEACEAE SCHIMP. 1856. previously published collection from the two countries (O’Shea, 1993). Apart from one Cryphaeaceae Schimp., Coroll. Bryol. Eur. 97. African taxon occurring in nine countries, the 1856 [‘1855’]. Type: Cryphaea D.Mohr in other 10 occurred in an average of 1.7 countries. F.Weber This particular profile is typical of unrevised genera in Africa, and indicative of a possible A brief review of the circumscription and need for revision (O’Shea, 1997), particularly systematics of the family, and the distinctions from related families (e.g. -

The Origin and Early Evolution of Vascular Plant Shoots and Leaves Rstb.Royalsocietypublishing.Org C
Downloaded from http://rstb.royalsocietypublishing.org/ on January 22, 2018 The origin and early evolution of vascular plant shoots and leaves rstb.royalsocietypublishing.org C. Jill Harrison 1 and Jennifer L. Morris 2 1School of Biological Sciences, and 2School of Earth Sciences, University of Bristol, 24 Tyndall Avenue, Bristol BS8 1TQ, UK Review CJH, 0000-0002-5228-600X; JLM, 0000-0002-7453-3841 Cite this article: Harrison CJ, Morris JL. 2017 The morphology of plant fossils from the Rhynie chert has generated long- standing questions about vascular plant shoot and leaf evolution, for The origin and early evolution of vascular plant instance, which morphologies were ancestral within land plants, when did shoots and leaves. Phil. Trans. R. Soc. B 373 : vascular plants first arise and did leaves have multiple evolutionary origins? 20160496. Recent advances combining insights from molecular phylogeny, palaeobotany http://dx.doi.org/10.1098/rstb.2016.0496 and evo–devo research address these questions and suggest the sequence of morphological innovation during vascular plant shoot and leaf evolution. The evidence pinpoints testable developmental and genetic hypotheses relat- Accepted: 11 August 2017 ing to the origin of branching and indeterminate shoot architectures prior to the evolution of leaves, and demonstrates underestimation of polyphyly in One contribution of 18 to a discussion meeting the evolution of leaves from branching forms in ‘telome theory’ hypotheses issue ‘The Rhynie cherts: our earliest terrestrial of leaf evolution. This review discusses fossil, developmental and genetic ecosystem revisited’. evidence relating to the evolution of vascular plant shoots and leaves in a phylogenetic framework. This article is part of a discussion meeting issue ‘The Rhynie cherts: our Subject Areas: earliest terrestrial ecosystem revisited’. -

Number of Living Species in Australia and the World
Numbers of Living Species in Australia and the World 2nd edition Arthur D. Chapman Australian Biodiversity Information Services australia’s nature Toowoomba, Australia there is more still to be discovered… Report for the Australian Biological Resources Study Canberra, Australia September 2009 CONTENTS Foreword 1 Insecta (insects) 23 Plants 43 Viruses 59 Arachnida Magnoliophyta (flowering plants) 43 Protoctista (mainly Introduction 2 (spiders, scorpions, etc) 26 Gymnosperms (Coniferophyta, Protozoa—others included Executive Summary 6 Pycnogonida (sea spiders) 28 Cycadophyta, Gnetophyta under fungi, algae, Myriapoda and Ginkgophyta) 45 Chromista, etc) 60 Detailed discussion by Group 12 (millipedes, centipedes) 29 Ferns and Allies 46 Chordates 13 Acknowledgements 63 Crustacea (crabs, lobsters, etc) 31 Bryophyta Mammalia (mammals) 13 Onychophora (velvet worms) 32 (mosses, liverworts, hornworts) 47 References 66 Aves (birds) 14 Hexapoda (proturans, springtails) 33 Plant Algae (including green Reptilia (reptiles) 15 Mollusca (molluscs, shellfish) 34 algae, red algae, glaucophytes) 49 Amphibia (frogs, etc) 16 Annelida (segmented worms) 35 Fungi 51 Pisces (fishes including Nematoda Fungi (excluding taxa Chondrichthyes and (nematodes, roundworms) 36 treated under Chromista Osteichthyes) 17 and Protoctista) 51 Acanthocephala Agnatha (hagfish, (thorny-headed worms) 37 Lichen-forming fungi 53 lampreys, slime eels) 18 Platyhelminthes (flat worms) 38 Others 54 Cephalochordata (lancelets) 19 Cnidaria (jellyfish, Prokaryota (Bacteria Tunicata or Urochordata sea anenomes, corals) 39 [Monera] of previous report) 54 (sea squirts, doliolids, salps) 20 Porifera (sponges) 40 Cyanophyta (Cyanobacteria) 55 Invertebrates 21 Other Invertebrates 41 Chromista (including some Hemichordata (hemichordates) 21 species previously included Echinodermata (starfish, under either algae or fungi) 56 sea cucumbers, etc) 22 FOREWORD In Australia and around the world, biodiversity is under huge Harnessing core science and knowledge bases, like and growing pressure. -

Getting to the Roots: a Developmental Genetic View of Root Anatomy and Function from Arabidopsis to Lycophytes
fpls-09-01410 September 21, 2018 Time: 17:3 # 1 REVIEW published: 25 September 2018 doi: 10.3389/fpls.2018.01410 Getting to the Roots: A Developmental Genetic View of Root Anatomy and Function From Arabidopsis to Lycophytes Frauke Augstein and Annelie Carlsbecker* Department of Organismal Biology, Physiological Botany and Linnean Centre for Plant Biology in Uppsala, Uppsala University, Uppsala, Sweden Roots attach plants to the ground and ensure efficient and selective uptake of water and nutrients. These functions are facilitated by the morphological and anatomical structures of the root, formed by the activity of the root apical meristem (RAM) and consecutive patterning and differentiation of specific tissues with distinct functions. Despite the importance of this plant organ, its evolutionary history is not clear, but fossils suggest that roots evolved at least twice, in the lycophyte (clubmosses and their allies) and in the euphyllophyte (ferns and seed plants) lineages. Both lycophyte and euphyllophyte roots grow indeterminately by the action of an apical meristem, which is protected by a root cap. They produce root hairs, and in most species the vascular stele is Edited by: guarded by a specialized endodermal cell layer. Hence, most of these traits must have Annette Becker, evolved independently in these lineages. This raises the question if the development Justus Liebig Universität Gießen, Germany of these apparently analogous tissues is regulated by distinct or homologous genes, Reviewed by: independently recruited from a common ancestor of lycophytes and euphyllophytes. Hongchang Cui, Currently, there are few studies of the genetic and molecular regulation of lycophyte Florida State University, United States and fern roots. -

Anthocerotophyta
Glime, J. M. 2017. Anthocerotophyta. Chapt. 2-8. In: Glime, J. M. Bryophyte Ecology. Volume 1. Physiological Ecology. Ebook 2-8-1 sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 5 June 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 2-8 ANTHOCEROTOPHYTA TABLE OF CONTENTS Anthocerotophyta ......................................................................................................................................... 2-8-2 Summary .................................................................................................................................................... 2-8-10 Acknowledgments ...................................................................................................................................... 2-8-10 Literature Cited .......................................................................................................................................... 2-8-10 2-8-2 Chapter 2-8: Anthocerotophyta CHAPTER 2-8 ANTHOCEROTOPHYTA Figure 1. Notothylas orbicularis thallus with involucres. Photo by Michael Lüth, with permission. Anthocerotophyta These plants, once placed among the bryophytes in the families. The second class is Leiosporocerotopsida, a Anthocerotae, now generally placed in the phylum class with one order, one family, and one genus. The genus Anthocerotophyta (hornworts, Figure 1), seem more Leiosporoceros differs from members of the class distantly related, and genetic evidence may even present -

Paleontological Resources at Grand Teton National Park, Northwestern Wyoming Vincent L
University of Wyoming National Park Service Research Center Annual Report Volume 22 22nd Annual Report, 1998 Article 7 1-1-1998 Paleontological Resources at Grand Teton National Park, Northwestern Wyoming Vincent L. Santucci National Park Service William P. Wall Georgia College and State University Follow this and additional works at: http://repository.uwyo.edu/uwnpsrc_reports Recommended Citation Santucci, Vincent L. and Wall, William P. (1998) "Paleontological Resources at Grand Teton National Park, Northwestern Wyoming," University of Wyoming National Park Service Research Center Annual Report: Vol. 22 , Article 7. Available at: http://repository.uwyo.edu/uwnpsrc_reports/vol22/iss1/7 This Grand Teton National Park Report is brought to you for free and open access by Wyoming Scholars Repository. It has been accepted for inclusion in University of Wyoming National Park Service Research Center Annual Report by an authorized editor of Wyoming Scholars Repository. For more information, please contact [email protected]. Santucci and Wall: Paleontological Resources at Grand Teton National Park, Northwest PALEONTOLOGICAL RESOURCES AT GRAND TETON NATIONAL PARK, NORTHWESTERN WYOMING + VINCENT L. SANTUCCI+ NATIONAL PARK SERVICE KEMMERER + WY WILLIAM P. WALL+ DEPARTMENT OF BIOLOGY GEORGIA COLLEGE AND STATE UNIVERSITY MILLEDGEVILLE + GA + ABSTRACT landscape, and though the last great ice masses melted 15 ,000 years ago, some re-established small Paleontological resources occur throughout glaciers still exist. the formations exposed in Grand Teton National Park. A comprehensive paleontological survey has This report provides a preliminary not been attempted previously at Grand Teton assessment of paleontological resources identified at National Park. Preliminary paleontologic resource Grand Teton National Park. data is given in this report in order to establish baseline data. -

Tansley Review Evolution of Development of Vascular Cambia and Secondary Growth
New Phytologist Review Tansley review Evolution of development of vascular cambia and secondary growth Author for correspondence: Rachel Spicer1 and Andrew Groover2 Andrew Groover 1The Rowland Institute at Harvard, Cambridge, MA, USA; 2Institute of Forest Genetics, Pacific Tel: +1 530 759 1738 Email: [email protected] Southwest Research Station, USDA Forest Service, Davis, CA, USA Received: 29 December 2009 Accepted: 14 February 2010 Contents Summary 577 V. Evolution of development approaches for the study 587 of secondary vascular growth I. Introduction 577 VI. Conclusions 589 II. Generalized function of vascular cambia and their 578 developmental and evolutionary origins Acknowledgements 589 III. Variation in secondary vascular growth in angiosperms 581 References 589 IV. Genes and mechanisms regulating secondary vascular 584 growth and their evolutionary origins Summary New Phytologist (2010) 186: 577–592 Secondary growth from vascular cambia results in radial, woody growth of stems. doi: 10.1111/j.1469-8137.2010.03236.x The innovation of secondary vascular development during plant evolution allowed the production of novel plant forms ranging from massive forest trees to flexible, Key words: forest trees, genomics, Populus, woody lianas. We present examples of the extensive phylogenetic variation in sec- wood anatomy, wood formation. ondary vascular growth and discuss current knowledge of genes that regulate the development of vascular cambia and woody tissues. From these foundations, we propose strategies for genomics-based research in the evolution of development, which is a next logical step in the study of secondary growth. I. Introduction this pattern characterizes most extant forest trees, significant variation exists among taxa, ranging from extinct woody Secondary vascular growth provides a means of radially lycopods and horsetails with unifacial cambia (Cichan & thickening and strengthening plant axes initiated during Taylor, 1990; Willis & McElwain, 2002), to angiosperms primary, or apical growth. -

University of Michigan University Library
CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY THE UNIVERSITY OF MICHIGAN VOL. XVIII, NO. 13, pp. 205-227 (6 pls.) OCTOBER11, 1963 t i THE FERN GENUS ACROSTICHUM IN THE EOCENE CLARNO FORMATION OF OREGON BY CHESTER A. ARNOLD and LYMAN H. DAUGHERTY FROM THE EDWARD PULTENEY WRIGHT MEMORIAL VOLUME Publication of this paper is made possible by the Federal-Mogul-Bower Bearings, Inc. Paleontology Research Fund MUSEUM OF PALEONTOLOGY THE UNIVERSITY OF MICHIGAN ANN ARBOR CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY Director: LEWISB. KELLUM The series of contributions from the Museum of Paleontology is a medium for the publication of papers based chiefly upon the collection in the Museum. When the number of pages issued is sufficient to make a volume, a title page and a table of contents will be sent to libraries on the mailing list, and to individuals upon request. A list of the separate papers may also be obtained. Correspondence v should be directed to the Museum of Paleontology, The University of Michigan, Ann Arbor, Michigan. VOLS. 11-XVII. Parts of volumes may be obtained if available. VOLUMEXVIII 1. Morphology and Taxonomy of the Cystoid Cheirocrinus anatiformis (Hall), by Robert V. Kesling. Pages 1-21, with 4 plates. 2. Ordovician Streptelasmid Rugose Corals from Michigan, by Erwin C. Sturnm. Pages 23-31, with 2 plates. 3. Paraconularia newberryi (Winchell) and other Lower Mississippian Conulariids from Michigan, Ohio, Indiana, and Iowa, by Egbert G. Driscoll. Pages 33-46, with 3 plates. 4. Two New Genera of Stricklandid Brachiopods, by A. J. Boucot and G. M. Ehlers. Pages 47-66, with 5 plates. -
A Guide to Native Plants in North Sydney Nurseries Who Supply Local Native Plants for the North Sydney Region
Live Local Plant Local a guide to native plants in North Sydney Nurseries who supply local native plants for the North Sydney region Ku-ring-gai Community Nursery Run through Ku-ring-gai Council. Ask for local plants for North Sydney area. 430 Mona Vale Road, St. Ives. Phone: (02) 9424 0376 / 0409 035 570 Tharwa Native Nursery Retail/Wholesale. Ask for local species for North Sydney area. 21 Myoora Road, Terry Hills. Phone: (02) 9450 1967 www.tubestocktharwanursery.com.au Wirreanda Nursery Indigenous species that Retail/Wholesale. Ask for local native species for North Sydney. make ideal garden plants 7 Wirreanda Road North, Ingleside. Phone: (02) 9450 1400 We can preserve and recreate some of North Sydney’s www.wirreandanursery.com.au unique native vegetation in our gardens by planting locally indigenous species. Many native species are Harvest Seeds & Native Plants becoming rare and our bushland is under threat from Retail/Wholesale. fragmentation, degradation, and the introduction of exotic Provenance is displayed. species. Planting locally not only benefits the environment 281 Mona Vale Road, Terry Hills. and native fauna, but is also beneficial to you, as these Phone: (02) 9450 2699 species require little watering, fertilising and maintenance. www.harvestseeds-nativeplants.com.au The selection of 30 indigenous species over the next few Indigo Native Nursery pages make ideal garden plants because they are hardy, Lot 57 Wattle Road, Ingleside. attractive, suitable for a variety of conditions and are easy Phone: (02) 9970 8709 to maintain. -

Australian Plants Society South East NSW Group Page 1
Gmail.com Australian Plants Society South East NSW Group Newsletter number 104 February 2015 Contacts: President, Margaret Lynch, [email protected] Secretary, Michele Pymble, [email protected] Newsletter editor, John Knight, [email protected] Corymbia maculata Spotted Gum and Macrozamia communis Burrawang Next Meeting SATURDAY 7th March 2015 Following on from a very successful start to the year, members are in for a special treat this month. We have been fortunate in securing for this meeting the leader of the Grevillea Study Group Mr. Peter Olde. We will meet at 10.30am at the Eurobodalla Regional Botanic Gardens Princes Highway, about 5km south of Batemans Bay After Peter’s presentation, and lunch at the Gardens, we will travel to Moruya for a look at some Grevilleas growing at Mark and Carolyn Noake’s garden, and then participate in a practical propagation session. Please bring morning tea and lunch. Those wishing to do so may purchase lunch from the Chefs Cap Café at the gardens. As we will walk around the Noake garden, comfortable walking shoes and a hat are advisable. There is plenty of seating at the ERBG, but you will need to put in a chair for use at Mark and Carolyn’s. See page 2 for details of these activities Future activities Due to Easter falling in the first week of April, the committee is discussing whether the April walk will be deferred till the 11th,. More on this next newsletter Our next meeting on May 2nd, is another treat for members. We will have to travel a bit, but Fern expert Kylie Stocks will let us in on the secrets of growing ferns successfully. -
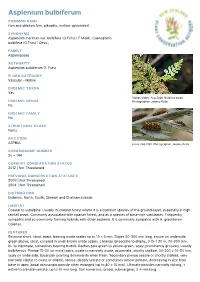
Asplenium Bulbiferum
Asplenium bulbiferum COMMON NAME Hen and chicken fern, pikopiko, mother spleenwort SYNONYMS Asplenium marinum var. bulbifera (G.Forst.) F.Muell.; Caenopteris bulbifera (G.Forst.) Desv.; FAMILY Aspleniaceae AUTHORITY Asplenium bulbiferum G. Forst. FLORA CATEGORY Vascular – Native ENDEMIC TAXON Yes Stokes Valley. Aug 2006. Bulbil on bulbil. ENDEMIC GENUS Photographer: Jeremy Rolfe No ENDEMIC FAMILY No STRUCTURAL CLASS Ferns NVS CODE ASPBUL sorus. Feb 1981. Photographer: Jeremy Rolfe CHROMOSOME NUMBER 2n = 144 CURRENT CONSERVATION STATUS 2012 | Not Threatened PREVIOUS CONSERVATION STATUSES 2009 | Not Threatened 2004 | Not Threatened DISTRIBUTION Endemic. North, South, Stewart and Chatham Islands HABITAT Coastal to subalpine. Usually in lowland forest where it is a common species of the ground-layer, especially in high rainfall areas. Commonly associated with riparian forest, and as a species of base-rich substrates. Frequently sympatric and so commonly forming hybrids with other asplenia. It is commonly sympatric with A. gracillimum Colenso. FEATURES Rhizome short, stout, erect, bearing ovate scales up to 15 × 5 mm. Stipes 50-300 mm long, brown on underside, green above, stout, covered in small brown ovate scales. Laminae lanceolate to elliptic, 0.15-1.20 m, 70-300 mm, bi- to tripinnate, sometimes bearing bulbils. Raches pale green to yellow-green, scaly, prominently grooved, usually bulbiferous. Pinnae 15-30 (or more) pairs, ovate to narrowly ovate, acuminate, shortly stalked, 30-200 × 10-50 mm, scaly on underside, basal pair pointing downwards when fresh. Secondary pinnae sessile or shortly stalked, very narrowly elliptic to ovate or elliptic, obtuse, deeply serrate or sometimes almost pinnate, decreasing in size from base to apex, basal acroscopic pinnule often enlarged (up to 40 × 10 mm).