Advances in Understanding and Managing Insect Pests of Forest Trees
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development of Single Nucleotide Polymorphism (SNP)
insects Article Development of Single Nucleotide Polymorphism (SNP) Markers for Analysis of Population Structure and Invasion Pathway in the Coconut Leaf Beetle Brontispa longissima (Gestro) Using Restriction Site-Associated DNA (RAD) Genotyping in Southern China Zhiming Chen 1,2,3, Guihua Wang 1,2 , Min Li 4, Zhengqiang Peng 5, Habib Ali 1,2,6, Lina Xu 1,2, Geoff M. Gurr 1,2,7,* and Youming Hou 1,2,* 1 State Key Laboratory of Ecological Pest Control of Fujian-Taiwan Crops, Fujian Agriculture and Forestry University, Fuzhou 350002, China; [email protected] (Z.C.); [email protected] (G.W.); [email protected] (H.A.); [email protected] (L.X.) 2 Fujian Provincial Key Laboratory of Insect Ecology, College of Plant Protection, Fujian Agriculture and Forestry University, Fuzhou 350002, China 3 Rongcheng Customs District of China, Fuzhou 350015, China 4 Technology Center of Fuzhou Customs District, Fuzhou 350000, China; [email protected] 5 Environment and Plant Protection Institute, Chinese Academy of Tropical Agricultural Sciences, Haikou 571101, China; [email protected] 6 Department of Entomology, University of Agriculture Faisalabd, Sub Campus Depalpur, Okara 56300, Pakistan 7 Graham Centre, Charles Sturt University, Orange NSW 2800, Australia * Correspondence: [email protected] (G.M.G.); [email protected] (Y.H.) Received: 13 February 2020; Accepted: 1 April 2020; Published: 7 April 2020 Abstract: Todetermine population genomic structure through high-throughput sequencing techniques has revolutionized research on non-model organisms. The coconut leaf beetle, Brontispa longissima (Gestro), is a widely distributed pest in Southern China. Here, we used restriction site-associated DNA (RAD) genotyping to assess the invasion pathway by detecting and estimating the degree of genetic differentiation among 51 B. -

The Invasive Alien Leaf Miner Cameraria Ohridella and the Native Tree Acer Pseudoplatanus: a Fatal Attraction?
1 The invasive alien leaf miner Cameraria ohridella and the native tree Acer pseudoplatanus: a fatal attraction? Christelle Per´ e†,´ Sylvie Augustin∗, Ted C. J. Turlings† and Marc Kenis CABI Europe-Switzerland, 2800 Del´emont, Switzerland, ∗INRA, UR 633 Zoologie Foresti`ere, 45000 Orl´eans, France and †Institute of Zoology, University of Neuchˆatel, 2009 Neuchˆatel, Switzerland Abstract 1 The horse-chestnut leaf miner Cameraria ohridella is an invasive moth in Europe and a serious pest of horse-chestnut Aesculus hippocastanum. The moth also occasionally attacks sycamore maple Acer pseudoplatanus, when situated beside infested horse-chestnuts. 2 The main objective of the present study was to provide an overview of the relationship between C. ohridella and A. pseudoplatanus and to determine whether C. ohridella has the potential to shift to this native tree. 3 In the field, females oviposit on different deciduous tree species. Although less frequently attacked than A. hippocastanum, A. pseudoplatanus was clearly preferred for oviposition over 12 other woody species investigated. 4 Surveys in Europe demonstrated that the majority of A. pseudoplatanus trees found beside infested A. hippocastanum had mines of C. ohridella, even though more than 70% of the larvae died within the first two instars. Attack rates and development success greatly varied from site to site. Attack levels on A. pseudoplatanus were not always correlated with those on A. hippocastanum, and mines on A. pseudoplatanus were sometimes observed beside weakly-infested A. hippocastanum. 5 Field observations, experimental exposure of A. pseudoplatanus saplings and rearing trials in a common garden study showed that individual trees may vary in their susceptibility to C. -

Cinara Cupressivora W Atson & Voegtlin, 1999 Other Scientific Names: Order and Family: Hemiptera: Aphididae Common Names: Giant Cypress Aphid; Cypress Aphid
O R E ST E ST PE C IE S R O FIL E F P S P November 2007 Cinara cupressivora W atson & Voegtlin, 1999 Other scientific names: Order and Family: Hemiptera: Aphididae Common names: giant cypress aphid; cypress aphid Cinara cupressivora is a significant pest of Cupressaceae species and has caused serious damage to naturally regenerating and planted forests in Africa, Europe, Latin America and the Caribbean and the Near East. It is believed to have originated on Cupressus sempervirens from eastern Greece to just south of the Caspian Sea (Watson et al., 1999). This pest has been recognized as a separate species for only a short time (Watson et al., 1999) and much of the information on its biology and ecology has been reported under the name Cinara cupressi. Cypress aphids (Photos: Bugwood.org – W .M . Ciesla, Forest Health M anagement International (left, centre); J.D. W ard, USDA Forest Service (right)) DISTRIBUTION Native: eastern Greece to just south of the Caspian Sea Introduced: Africa: Burundi (1988), Democratic Republic of Congo, Ethiopia (2004), Kenya (1990), Malawi (1986), Mauritius (1999), Morocco, Rwanda (1989), South Africa (1993), Uganda (1989), United Republic of Tanzania (1988), Zambia (1985), Zimbabwe (1989) Europe: France, Italy, Spain, United Kingdom Latin America and Caribbean: Chile (2003), Colombia Near East: Jordan, Syria, Turkey, Yemen IDENTIFICATION Giant conifer aphid adults are typically 2-5 mm in length, dark brown in colour with long legs (Ciesla, 2003a). Their bodies are sometimes covered with a powdery wax. They typically occur in colonies of 20-80 adults and nymphs on the branches of host trees (Ciesla, 1991). -

Scope: Munis Entomology & Zoology Publishes a Wide Variety of Papers
732 _____________Mun. Ent. Zool. Vol. 7, No. 2, June 2012__________ STRUCTURE OF LEPIDOPTEROCENOSES ON OAKS QUERCUS DALECHAMPII AND Q. CERRIS IN CENTRAL EUROPE AND ESTIMATION OF THE MOST IMPORTANT SPECIES Miroslav Kulfan* * Department of Ecology, Faculty of Natural Sciences, Comenius University, Mlynská dolina B-1, SK-84215 Bratislava, SLOVAKIA. E-mail: [email protected] [Kulfan, M. 2012. Structure of lepidopterocenoses on oaks Quercus dalechampii and Q. cerris in Central Europe and estimation of the most important species. Munis Entomology & Zoology, 7 (2): 732-741] ABSTRACT: On the basis of lepidopterous larvae a total of 96 species on Quercus dalechampii and 58 species on Q. cerris were recorded in 10 study plots of Malé Karpaty and Trnavská pahorkatina hills. The families Geometridae, Noctuidae and Tortricidae encompassed the highest number of found species. The most recorded species belonged to the trophic group of generalists. On the basis of total abundance of lepidopterous larvae found on Q. dalechampii from all the study plots the most abundant species was evidently Operophtera brumata. The most abundant species on Q. cerris was Cyclophora ruficiliaria. Based on estimated oak leaf area consumed by a larva it is shown that Lymantria dispar was the most important leaf-chewing species of both Q. dalechampii and Q. cerris. KEY WORDS: Slovakia, Quercus dalechampii, Q. cerris, the most important species. About 300 Lepidoptera species are known to damage the assimilation tissue of oaks in Central Europe (Patočka, 1954, 1980; Patočka et al.1999; Reiprich, 2001). Lepidoptera larvae are shown to be the most important group of oak defoliators (Patočka et al., 1962, 1999). -

Thesis.Pdf (3.979Mb)
FACULTY OF BIOSCIENCES, FISHERIES AND ECONOMICS DEPARTMENT OF ARCTIC AND MARINE BIOLOGY Cyclically outbreaking geometrid moths in sub-arctic mountain birch forest: the organization and impacts of their interactions with animal communities — Ole Petter Laksforsmo Vindstad A dissertation for the degree of Philosophiae Doctor – October 2014 Cyclically outbreaking geometrid moths in sub-arctic mountain birch forest: the organization and impacts of their interactions with animal communities Ole Petter Laksforsmo Vindstad A dissertation for the degree of Philosophiae Doctor University of Tromsø – The arctic university of Norway Faculty of Biosciences, Fisheries and Economics Department of Arctic and Marine Biology Autumn 2014 1 Dedicated to everyone who has helped me along the way 2 Supervisors Professor Rolf Anker Ims1 Senior researcher Jane Uhd Jepsen2 1 Department of Arctic and Marine Biology, University of Tromsø, Tromsø, Norway 2 Norwegian Institute for Nature Research, Fram Centre, Tromsø, Norway Cover photos Front cover – Larvae of Epirrita autumnata feeding on mountain birch during a moth outbreak in northern Norway. Photo: Moritz Klinghardt Study I – Portrait of Agrypon flaveolatum. One of the most important larval parasitoid species in study I. Photo: Ole Petter Laksforsmo Vindstad Study II – Carcass of an Operophtera brumata larva, standing over the cocoon of its killer, the parasitoid group Protapanteles anchisiades/P. immunis/Cotesia salebrosa. Photo: Ole Petter Laksforsmo Vindstad Study III – Larva of the parasitoid group Phobocampe sp./Sinophorus crassifemur emerging from Agriopis aurantiaria host larva. Photo: Tino Schott Study IV – An area of healthy mountain birch forest, representative for the undamaged sampling sites in study IV and V. Photo: Jakob Iglhaut Study V – An area of mountain birch forest that has been heavily damaged by a moth outbreak, representative for the damaged sampling sites in study IV and V. -

Cinara Cupressivora
62 Global review of forest pests and diseases Cinara cupressivora Order and Family: Hemiptera: Aphididae Common names: giant cypress aphid; cypress aphid Cinara cupressivora Watson & Voegtlin, 1999 is a significant pest of Cupressaceae species and has caused serious damage to naturally regenerated and planted forests in Africa, Europe, Latin America and the Caribbean and the Near East. It is believed to have originated on Cupressus sempervirens from eastern Greece to just south of the Caspian Sea (Watson et al., 1999). This pest has been recognized as a separate species for only a short time (Watson et al., 1999) and much of the information on its biology and ecology has been reported under the name Cinara cupressi. BUGWOOD.ORG/W.M. CIESLA/3948001 BUGWOOD.ORG/W.M. CIESLA/3948002 BUGWOOD.ORG/W.M. BUGWOOD.ORG/J.D. WARD/2912011 Cypress aphids DISTRIBUTION Native: Europe and the Near East: eastern Greece to Islamic Republic of Iran Introduced: Africa: Burundi (1988), Democratic Republic of Congo, Ethiopia (2004), Kenya (1990), Malawi (1986), Mauritius (1999), Morocco, Rwanda (1989), South Africa (1993), Uganda (1989), United Republic of Tanzania (1988), Zambia (1985), Zimbabwe (1989) Europe: France, Italy, Spain, United Kingdom Latin America and Caribbean: Chile (2003), Colombia Near East: Jordan, the Syrian Arab Republic, Turkey, Yemen IDENTIFICATION Giant conifer aphid adults are typically 2 to 5 mm in length, dark brown in colour with long legs (Ciesla, 2003a). Their bodies are sometimes covered with a powdery wax. They typically occur in colonies of 20 to 80 adults and nymphs on the branches of host trees (Ciesla, 1991). -

Cameraria Ohridella and OTHER PESTS E
POTENTIAL OF THE STRAIN OF ENTOMOPATHOGENIC FUNGUS Isaria fumosorosea CCM 8367 AS A BIOLOGICAL CONTROL AGENT AGAINST Cameraria ohridella AND OTHER PESTS E. Prenerova´ 1, R. Zemek2, F. Weyda2, L. Volter2, M. Awad2 and H. M. Hussein2 1Plant Protection Laboratory Olesnˇ a,´ Olesnˇ a´ 87, Bernartice u Milevska, Czech Republic; [email protected] 2Institute of Entomology, Biology Centre AS CR, Branisovskˇ a´ 31, Ceskˇ e´ Budejovice,ˇ Czech Republic; [email protected] The effect on Spodoptera littoralis larvae Background Results of bioassays revealed higher virulence of CCM 8367 strain compared to PreFeRalR WG strain (Figs. 6 and 7). Entomopathogenic fungus Isaria fumosorosea (syn. Paecilo- myces fumosoroseus) (WIZE) Brown & Smith (Deuteromycota) is potentially usefull for the biocontrol of economically impor- tant agricultural and forest insect pests. Selection of effective, highly virulent strain is prerequisite for development of success- full biopesticide. Our strain of I. fumosorosea was isolated in the Czech Re- public from the horse chestnut leaf miner, Cameraria ohridella FIGURE 2: Cumulative mortality of C. ohridella pupae (Fig. 1), an invasive species that has spread rapidly through treated with I. fumosorosea blastospores. Red line: CCM central and Western Europe [1]. The strain is deposited under 8367; blue line: PreFeRalR WG; green line: control; number CCM 8367 (CCEFO.011.PFR) as a patent culture in n=200. the Czech Collection of Microorganisms in Brno. The effect on Cameraria ohridella pupae in horse chestnut leaves FIGURE 6: Cumulative mortality of S. littoralis larvae treated with I. fumosorosea blastospores. Red line: CCM Mortality of C. ohridella pupae in leaf samples was significantly 8367; blue line: PreFeRalR WG; green line: control; n=30. -

Drought and Moisture Availability and Recent Western Spruce Budworm Outbreaks in the Western United States
Article Drought and Moisture Availability and Recent Western Spruce Budworm Outbreaks in the Western United States Bingbing Xu 1, Jeffrey A. Hicke 2,* and John T. Abatzoglou 2 1 Environmental Science Program, University of Idaho, Moscow, ID 83844, USA; [email protected] 2 Department of Geography, University of Idaho, Moscow, ID 83844, USA; [email protected] * Correspondence: [email protected]; Tel.: +01-208-885-6240 Received: 27 February 2019; Accepted: 19 April 2019; Published: 24 April 2019 Abstract: Western spruce budworm (WSBW) is a common defoliating insect that has caused extensive damage and mortality to a number of tree species across the western United States (US). Past studies have linked outbreaks of WSBW to increased moisture stress of host trees in the Northwest and decreased moisture stress in the Southwest. Our study analyzed seasonal drought stress metrics with WSBW outbreaks within Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) forests in the western US during 1997–2015. Superposed epoch analysis and defoliation area growth rates (representing insect population growth rates) were assessed to quantify the drought conditions associated with the initiation and continuation of outbreaks, respectively. We found that multiple years of drought occurred prior to and during outbreak initiation in the Northwest, and that outbreak initiation in the Southwest was associated with only weak drought or neutral conditions. During the outbreak continuation stage, there was a weak positive correlation between May moisture availability and defoliation area growth rates in the Southwest (R2 = 0.12), but no clear relationship was identified in the Northwest. Increased frequency of summer droughts such as these expected from climate change may increase WSBW outbreaks and promote tree dieoff. -

Goldspotted Oak Borer T.W
Forest Insect & Disease Leaflet 183 March 2015 U.S. Department of Agriculture • Forest Service Goldspotted Oak Borer T.W. Coleman1, M.I. Jones2, S.L. Smith3, R.C. Venette4, M.L. Flint5, and S.J. Seybold 6 The goldspotted oak borer (GSOB), New Mexico, and southwestern Texas. Agrilus auroguttatus Schaeffer Specimens of GSOB have only been (Coleoptera: Buprestidae) (Figure collected from Arizona, California, 1), is a flatheaded phloem- and wood and Mexico. In southeastern Arizona, borer that infests and kills several GSOB feeds primarily on Q. emoryi, species of oak (Fagaceae: Quercus) in and silverleaf oak, Q. hypoleucoides A. California. One or more populations Camus (both Section Lobatae). Larval of GSOB were likely introduced via feeding injures the phloem and outer infested firewood into San Diego xylem of these red oak species, with County, California from the native most feeding activity and occasional range in southeastern Arizona. Since cases of tree mortality noted in large- its introduction to California, GSOB has expanded its range and has killed red oaks (Quercus Section Lobatae) nearly continuously across public and private lands (Figure 2). Distribution and Hosts The native distribution of GSOB likely coincides with that of Emory oak, Q. emoryi Torrey, including the Coronado Figure 1. Adult goldspotted oak borer, Agrilus National Forest in southeastern auroguttatus, an exotic insect threatening red Arizona and floristically related oaks in California (Adults are approximately regions in northern Mexico, southern 0.35 inches long by 0.08 inches wide). 1Entomologist, USDA Forest Service, Forest Health Protection, San Bernardino, CA; 2Entomologist, Dept. of Environmental Science and Forestry, Syracuse University, Syracuse, NY; 3Entomologist, USDA Forest Service, Forest Health Protection, Susanville, CA; 4Research Biologist, USDA Forest Service, Northern Research Station, St. -

Ecological and Chemical Aspects of White Oak Decline and Sudden Oak Death, Two Syndromes Associated with Phytophthora Spp
ECOLOGICAL AND CHEMICAL ASPECTS OF WHITE OAK DECLINE AND SUDDEN OAK DEATH, TWO SYNDROMES ASSOCIATED WITH PHYTOPHTHORA SPP. A Thesis Presented in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Graduate School of The Ohio State University By Annemarie Margaret Nagle Graduate Program in Plant Pathology The Ohio State University 2009 *** Thesis Committee: Pierluigi (Enrico) Bonello, Advisor Laurence V. Madden Robert P. Long Dennis J. Lewandowski Copyright by: Annemarie Margaret Nagle 2009 ABSTRACT Phytophthora spp., especially invasives, are endangering forests globally. P. ramorum causes lethal canker diseases on coast live oak (CLO) and tanoak, and inoculation studies have demonstrated pathogenicity on other North American oak species, particularly those in the red oak group such as northern red oak (NRO). No practical controls are available for this disease, and characterization of natural resistance is highly desirable. Variation in resistance to P. ramorum has been observed in CLO in both naturally infected trees and controlled inoculations. Previous studies suggested that phloem phenolic chemistry may play a role in induced defense responses to P. ramorum in CLO (Ockels et al. 2007) but did not establish a relationship between these defense responses and actual resistance. Here we describe investigations aiming to elucidate the role of constitutive phenolics in resistance by quantifying relationships between concentrations of individual compounds, total phenolics, and actual resistance in CLO and NRO. Four experiments were conducted. In the first, we used cohorts of CLOs previously characterized as relatively resistant (R) or susceptible (S). Constitutive (pre-inoculation) phenolics were extracted from R and S branches on three different dates. -

The Forestry Commission's Contingency Plan
Western Spruce Budworm (Choristoneura freemani), Eastern Spruce Budworm (Choristoneura fumiferana) Black-Headed Budworm (Acleris gloverana and Acleris variana) – Contingency Plan Combined Budworm: Draft contingency plan INTRODUCTION 1. Serious or significant pests require strategic-level plans developed at a national level describing the overall aim and high-level objectives to be achieved, and setting out the response strategy for eradicating or containing outbreaks. 2. The UK Plant Health Risk Group (PHRG) has commissioned, following identification by the UK Plant Health Risk Register, pest-specific contingency plans for those pests which pose the greatest risk and require stakeholder consultation. 3. The purpose of these pest-specific contingency plans is to ensure a rapid and effective response to outbreaks of the pests or diseases described. They are designed to help government agencies anticipate, assess, prepare for, prevent, respond to and recover from pest and disease outbreaks. 4. Contingency planning starts with the anticipation and assessment of potential threats, includes preparation and response, and finishes with recovery. Anticipate 5. Gathering information and intelligence about the pest, including surveillance and horizon scanning. Assess 6. Identifying concerns and preparing plans. 7. Setting outbreak management objectives Prepare 8. Ensuring staff and stakeholders are familiar with the pest. Response 9. Identifying the requirements for either containing or eradicating the pest or disease, including work to determine success. 10. The Defra Contingency Plan for Plant Health in England (in draft) gives details of the teams and organisations involved in pest and disease response in England, and their responsibilities and governance. It also 2 | Combined budworm contingency plan | Dafni Nianiaka | 24/01/2017 Combined Budworm: Draft contingency plan describes how these teams and organisations will work together in the event of an outbreak of a plant health pest. -
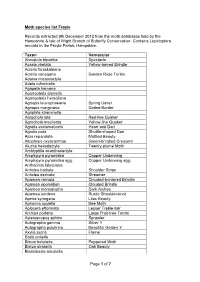
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera