Reptile-Like Physiology in Early Jurassic Stem-Mammals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reptile-Like Physiology in Early Jurassic Stem-Mammals
bioRxiv preprint doi: https://doi.org/10.1101/785360; this version posted October 10, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Title: Reptile-like physiology in Early Jurassic stem-mammals Authors: Elis Newham1*, Pamela G. Gill2,3*, Philippa Brewer3, Michael J. Benton2, Vincent Fernandez4,5, Neil J. Gostling6, David Haberthür7, Jukka Jernvall8, Tuomas Kankanpää9, Aki 5 Kallonen10, Charles Navarro2, Alexandra Pacureanu5, Berit Zeller-Plumhoff11, Kelly Richards12, Kate Robson-Brown13, Philipp Schneider14, Heikki Suhonen10, Paul Tafforeau5, Katherine Williams14, & Ian J. Corfe8*. Affiliations: 10 1School of Physiology, Pharmacology & Neuroscience, University of Bristol, Bristol, UK. 2School of Earth Sciences, University of Bristol, Bristol, UK. 3Earth Science Department, The Natural History Museum, London, UK. 4Core Research Laboratories, The Natural History Museum, London, UK. 5European Synchrotron Radiation Facility, Grenoble, France. 15 6School of Biological Sciences, University of Southampton, Southampton, UK. 7Institute of Anatomy, University of Bern, Bern, Switzerland. 8Institute of Biotechnology, University of Helsinki, Helsinki, Finland. 9Department of Agricultural Sciences, University of Helsinki, Helsinki, Finland. 10Department of Physics, University of Helsinki, Helsinki, Finland. 20 11Helmholtz-Zentrum Geesthacht, Zentrum für Material-und Küstenforschung GmbH Germany. 12Oxford University Museum of Natural History, Oxford, OX1 3PW, UK. 1 bioRxiv preprint doi: https://doi.org/10.1101/785360; this version posted October 10, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 13Department of Anthropology and Archaeology, University of Bristol, Bristol, UK. 14Faculty of Engineering and Physical Sciences, University of Southampton, Southampton, UK. -

Micheli Stefanello
UNIVERSIDADE FEDERAL DE SANTA MARIA CENTRO DE CIÊNCIAS NATURAIS E EXATAS PROGRAMA DE PÓS-GRADUAÇÃO EM BIODIVERSIDADE ANIMAL Micheli Stefanello DESCRIÇÃO E FILOGENIA DE UM NOVO ESPÉCIME DE CINODONTE PROBAINOGNÁTIO DO TRIÁSSICO SUL-BRASILEIRO Santa Maria, RS 2018 Micheli Stefanello DESCRIÇÃO E FILOGENIA DE UM NOVO ESPÉCIME DE CINODONTE PROBAINOGNÁTIO DO TRIÁSSICO SUL-BRASILEIRO Dissertação apresentada ao Curso de Mestrado do Programa de Pós-Graduação em Biodiversidade Animal, Área de Concentração em Sistemática e Biologia Evolutiva, da Universidade Federal de Santa Maria (UFSM, RS), como requisito parcial para obtenção do grau de Mestre em Ciências Biológicas – Área Biodiversidade Animal. Orientador: Prof. Dr. Sérgio Dias da Silva Santa Maria, RS 2018 Micheli Stefanello DESCRIÇÃO E FILOGENIA DE UM NOVO ESPÉCIME DE CINODONTE PROBAINOGNÁTIO DO TRIÁSSICO SUL-BRASILEIRO Dissertação apresentada ao Curso de Mestrado do Programa de Pós-Graduação em Biodiversidade Animal, Área de Concentração em Sistemática e Biologia Evolutiva, da Universidade Federal de Santa Maria (UFSM, RS), como requisito parcial para obtenção do grau de Mestre em Ciências Biológicas – Área Biodiversidade Animal. Aprovada em 28 de fevereiro de 2018: Santa Maria, RS 2018 AGRADECIMENTOS Ao meu orientador, Dr. Sérgio Dias da Silva, pela orientação, por todo o tempo despendido ao longo desse mestrado e por possibilitar meu aprimoramento na área a qual tenho apreço. Aos colegas do Centro de Apoio à Pesquisa Paleontológica da Quarta Colônia da Universidade Federal de Santa Maria (CAPPA/UFSM) e do Laboratório de Paleobiodiversidade Triássica, dessa mesma instituição, pela convivência e por terem ajudado-me de diferentes formas ao longo do mestrado. Em especial, ao colega Rodrigo Temp Müller, pela coleta do espécime (objeto de estudo dessa dissertação), por toda a ajuda com o TNT e com as figuras, e por auxiliar-me de inúmeras formas. -

Behavioral and Physiological Thermoregulation of Atacama Desert-Dwelling Liolaemus Lizards
Écoscience ISSN: 1195-6860 (Print) 2376-7626 (Online) Journal homepage: http://www.tandfonline.com/loi/teco20 Behavioral and physiological thermoregulation of Atacama desert-dwelling Liolaemus lizards Antonieta Labra, Mauricio Soto-Gamboa & Francisco Bozinovic To cite this article: Antonieta Labra, Mauricio Soto-Gamboa & Francisco Bozinovic (2001) Behavioral and physiological thermoregulation of Atacama desert-dwelling Liolaemus lizards, Écoscience, 8:4, 413-420, DOI: 10.1080/11956860.2001.11682669 To link to this article: https://doi.org/10.1080/11956860.2001.11682669 Published online: 23 Mar 2016. Submit your article to this journal Article views: 27 View related articles Citing articles: 17 View citing articles Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=teco20 Download by: [Pontificia Universidad Catolica de Chile] Date: 26 December 2017, At: 11:07 CoSCIENCE E 8 (4) : 413-420 (2001) Behavioral and physiological thermoregulation of Atacama desert-dwelling Liolaemus lizards1 Antonieta LABRA2, Mauricio SOTO-GAMBOA & Francisco BOZINOVIC, Departamento de Ecología, Facultad de Ciencias Biológicas, P. Universidad Católica de Chile, Casilla 114-D, Santiago, C.P. 6513677, Chile, e-mail: [email protected] Abstract: The behavioral and physiological thermoregulation of three Atacama desert-dwelling Liolaemus lizards was studied and compared with previous data on Liolaemus from other ecosystems. The thermoregulatory mechanisms of the desert-dwelling species differed from those of the others, a consequence of differences in the habitat structure of the species. Desert species have higher critical thermal minima and, contrary to expectations, lower selected body temperatures than Liolaemus from Mediterranean environments. Results of the rates of thermal time constants suggest mechanisms to cope with the fast decrease of environmental temperature that occurs in the desert during the mid-afternoons. -

Female Reproductive Biology of the Lizards <I>Liolaemus</I> <I
Volume 25 (April 2015), 101–108 FULL PAPER Herpetological Journal Published by the British Female reproductive biology of the lizards Liolaemus Herpetological Society sarmientoi and L. magellanicus from the southern end of the world Jimena B. Fernández1, Marlin Medina2, Erika L. Kubisch1, Amanda A. Manero3, J. Alejandro Scolaro4 & Nora R. Ibargüengoytía1 1INIBIOMA - CONICET. Departamento de Zoología, Laboratorio de Ecofisiología e Historia de vida de Reptiles, Centro Regional Universitario Bariloche, Universidad Nacional del Comahue, San Carlos de Bariloche (8400), Río Negro, Argentina 2CIEMEP-CONICET. Centro de Investigación en Ecología de Montaña y Estepa Patagónica. Departamento de Biología, Facultad de Ciencias Naturales, Universidad Nacional de la Patagonia San Juan Bosco, Esquel (9200), Chubut, Argentina 3Departamento de Ciencias Exactas y Naturales, Universidad Nacional de la Patagonia Austral, Río Gallegos (9400), Santa Cruz, Argentina 4CENPAT-CONICET, Puerto Madryn y Facultad de Ciencias Naturales, Universidad Nacional de la Patagonia San Juan Bosco, Trelew (9100), Chubut, Argentina Lizards that live in the harsh climate of the Argentinean Patagonia (40°–53° S) are active for a period restricted to spring and summer when vitellogenesis, pregnancy and birth take place. Herein, we present data on the female reproductive cycle, body size at sexual maturity, litter size and fat-body cycle of one of the world's southernmost reptiles, Liolaemus sarmientoi. We also provide preliminary data on the reproductive cycle of a sympatric species, L. magellanicus. Females of both species start vitellogenesis in late spring, probably arrested or continued at very low rates during brumation resumed in the spring of the next year. Pregnancy starts in spring and births of L. -

Characterization of Arm Autotomy in the Octopus, Abdopus Aculeatus (D’Orbigny, 1834)
Characterization of Arm Autotomy in the Octopus, Abdopus aculeatus (d’Orbigny, 1834) By Jean Sagman Alupay A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Integrative Biology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Roy L. Caldwell, Chair Professor David Lindberg Professor Damian Elias Fall 2013 ABSTRACT Characterization of Arm Autotomy in the Octopus, Abdopus aculeatus (d’Orbigny, 1834) By Jean Sagman Alupay Doctor of Philosophy in Integrative Biology University of California, Berkeley Professor Roy L. Caldwell, Chair Autotomy is the shedding of a body part as a means of secondary defense against a predator that has already made contact with the organism. This defense mechanism has been widely studied in a few model taxa, specifically lizards, a few groups of arthropods, and some echinoderms. All of these model organisms have a hard endo- or exo-skeleton surrounding the autotomized body part. There are several animals that are capable of autotomizing a limb but do not exhibit the same biological trends that these model organisms have in common. As a result, the mechanisms that underlie autotomy in the hard-bodied animals may not apply for soft bodied organisms. A behavioral ecology approach was used to study arm autotomy in the octopus, Abdopus aculeatus. Investigations concentrated on understanding the mechanistic underpinnings and adaptive value of autotomy in this soft-bodied animal. A. aculeatus was observed in the field on Mactan Island, Philippines in the dry and wet seasons, and compared with populations previously studied in Indonesia. -

Femur of a Morganucodontid Mammal from the Middle Jurassic of Central Russia
Femur of a morganucodontid mammal from the Middle Jurassic of Central Russia PETR P. GAMBARYAN and ALEXANDER 0.AVERIANOV Gambaryan, P.P. & Averianov, A.O. 2001. Femur of a morganucodontid mammal from the Middle Jurassic of Central Russia. -Acta Palaeontologica Polonica 46,1,99-112. We describe a nearly complete mammalian femur from the Middle Jurassic (upper Bathonian) from Peski quarry, situated some 100 km south east of Moscow, central Rus- sia. It is similar to the femora of Morganucodontidae in having a globular femoral head, separated from the greater trochanter and reflected dorsally, fovea capitis present, both trochanters triangular and located on the same plane, distal end flat, mediolaterally expanded, and somewhat bent ventrally, and in the shape and proportions of distal condyles. It is referred to as Morganucodontidae gen. et sp. indet. It is the first representa- tive of this group of mammals in Eastern Europe from the third Mesozoic mammal local- ity discovered in Russia. Exquisite preservation of the bone surface allowed us to recon- struct partial hind limb musculature. We reconstruct m. iliopsoas as inserting on the ridge, which starts at the lesser trochanter and extends along the medial femoral margin for more than half of the femur length. On this basis we conclude that the mode of loco- motion of the Peski morganucodontid was similar to that of modern echidnas. During the propulsive phase the femur did not retract and the step elongation was provided by pronation of the femur. Key words : Mammalia, Morganucodontidae, femur, anatomy, locomotion, Jurassic, Russia. Petr P. Gambaryan [[email protected]] and Alexander 0. -

Sexual Dimorphism in Perissodactyl Rhinocerotid Chilotherium Wimani from the Late Miocene of the Linxia Basin (Gansu, China)
Sexual dimorphism in perissodactyl rhinocerotid Chilotherium wimani from the late Miocene of the Linxia Basin (Gansu, China) SHAOKUN CHEN, TAO DENG, SUKUAN HOU, QINQIN SHI, and LIBO PANG Chen, S., Deng, T., Hou, S., Shi, Q., and Pang, L. 2010. Sexual dimorphism in perissodactyl rhinocerotid Chilotherium wimani from the late Miocene of the Linxia Basin (Gansu, China). Acta Palaeontologica Polonica 55 (4): 587–597. Sexual dimorphism is reviewed and described in adult skulls of Chilotherium wimani from the Linxia Basin. Via the anal− ysis and comparison, several very significant sexually dimorphic features are recognized. Tusks (i2), symphysis and oc− cipital surface are larger in males. Sexual dimorphism in the mandible is significant. The anterior mandibular morphology is more sexually dimorphic than the posterior part. The most clearly dimorphic character is i2 length, and this is consistent with intrasexual competition where males invest large amounts of energy jousting with each other. The molar length, the height and the area of the occipital surface are correlated with body mass, and body mass sexual dimorphism is compared. Society behavior and paleoecology of C. wimani are different from most extinct or extant rhinos. M/F ratio indicates that the mortality of young males is higher than females. According to the suite of dimorphic features of the skull of C. wimani, the tentative sex discriminant functions are set up in order to identify the gender of the skulls. Key words: Mammalia, Perissodactyla, Chilotherium wimani, sexual dimorphism, statistics, late Miocene, China. Shaokun Chen [[email protected]], Chongqing Three Gorges Institute of Paleoanthropology, China Three Gorges Museum, 236 Ren−Min Road, Chongqing 400015, China and Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, 142 Xi−Zhi−Men−Wai Street, P.O. -

The Origin of Mammals: a Study of Some Important Fossils
CEN Tech. J., vol. 7(2), 1993, pp. 122–139 The Origin of Mammals: A Study of Some Important Fossils A. W. MEHLERT ABSTRACT For many years evolutionists have pointed to the alleged transition of reptiles to mammals as being the best example of the natural origin of a major taxon. At first sight such claims appear to have considerable justification, but when the fossil evidence is closely examined it can be shown that there are some grave deficiencies, and the claim is not only factually unproved but also contains a number of dubious assumptions. It is therefore proposed to subject certain of the key fossils to more scrutiny. INTRODUCTION AND BRIEF OVERVIEW therapsids of later Permian and Triassic ‘times’. It is supposedly within the order Therapsida (suborder In 1966 Olson wrote, Theriodontia), that we find the subgroups to which the ‘The reptilian-mammalian transition has by far the immediate ancestors of mammals are assigned (see Table finest record of showing the origin of a new 24). class.’ (Emphasis added.)1 Almost all authorities are convinced that the immediate Kemp in 1982 had the same view.2 If it can be shown that reptilian ancestors of the early mammals are to be found in such a claim is not as strong as Olson and Kemp believe, the infraorders Cynodontia, Tritylodontia and Ictidosauria, then by implication, the evolutionary origin of all other although all six theriodont subgroups contain fossils which classes in the animal and plant kingdoms must be even more appear to bear at least some mammalian-like features. doubtful. -

Osteohistology of Late Triassic Prozostrodontian Cynodonts from Brazil
Osteohistology of Late Triassic prozostrodontian cynodonts from Brazil Jennifer Botha-Brink1,2, Marina Bento Soares3 and Agustín G. Martinelli3 1 Department of Karoo Palaeontology, National Museum, Bloemfontein, South Africa 2 Department of Zoology and Entomology, University of the Free State, Bloemfontein, South Africa 3 Departamento de Paleontologia e Estratigrafia, Instituto de Geociências, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil ABSTRACT The Prozostrodontia includes a group of Late Triassic-Early Cretaceous eucynodonts plus the clade Mammaliaformes, in which Mammalia is nested. Analysing their growth patterns is thus important for understanding the evolution of mammalian life histories. Obtaining material for osteohistological analysis is difficult due to the rare and delicate nature of most of the prozostrodontian taxa, much of which comprises mostly of crania or sometimes even only teeth. Here we present a rare opportunity to observe the osteohistology of several postcranial elements of the basal prozostrodontid Prozostrodon brasiliensis, the tritheledontid Irajatherium hernandezi, and the brasilodontids Brasilodon quadrangularis and Brasilitherium riograndensis from the Late Triassic of Brazil (Santa Maria Supersequence). Prozostrodon and Irajatherium reveal similar growth patterns of rapid early growth with annual interruptions later in ontogeny. These interruptions are associated with wide zones of slow growing bone tissue. Brasilodon and Brasilitherium exhibit a mixture of woven-fibered bone tissue and slower growing parallel-fibered and lamellar bone. The slower growing bone tissues are present even during early ontogeny. The relatively slower growth in Brasilodon and Brasilitherium may be related to their small body size compared to Prozostrodon and Irajatherium. These brasilodontids also exhibit osteohistological similarities with the Late Triassic/Early Jurassic mammaliaform Morganucodon and the Late Cretaceous multituberculate mammals Kryptobaatar and Nemegtbaatar. -

Early Cretaceous Amphilestid ('Triconodont') Mammals from Mongolia
Early Cretaceous amphilestid ('triconodont') mammals from Mongolia ZOFIAKIELAN-JAWOROWSKA and DEMBERLYIN DASHZEVEG Kielan-Jaworowską Z. &Daslueveg, D. 1998. Early Cretaceous amphilestid (.tricono- dont') mammals from Mongotia. - Acta Pal.aeontol.ogicaPolonica,43,3, 413438. Asmall collection of ?Aptianor ?Albian amphilestid('triconodont') mammals consisting of incomplete dentaries and maxillae with teeth, from the Khoboor localiĘ Guchin Us counĘ in Mongolia, is described. Grchinodon Troftmov' 1978 is regarded a junior subjective synonym of GobiconodonTroftmov, 1978. Heavier wear of the molariforms M3 andM4than of themore anteriorone-M2 in Gobiconodonborissiaki gives indirect evidence formolariformreplacement in this taxon. The interlocking mechanismbetween lower molariforms n Gobiconodon is of the pattern seen in Kuchneotherium and Ttnodon. The ińterlocking mechanism and the type of occlusion ally Amphilestidae with Kuehneotheriidae, from which they differ in having lower molariforms with main cusps aligned and the dentary-squamosal jaw joint (double jaw joint in Kuehneotheńdae). The main cusps in upper molariforms M3-M5 of Gobiconodon, however, show incipient tńangular arrangement. The paper gives some support to Mills' idea on the therian affinities of the Amphilestidae, although it cannot be excluded that the characters that unite the two groups developed in parallel. Because of scanty material and arnbiguĘ we assign the Amphilestidae to order incertae sedis. Key words : Mammali4 .triconodonts', Amphilestidae, Kuehneotheriidae, Early Cretaceous, Mongolia. Zofia Kiel,an-Jaworowska [zkielnn@twarda,pan.pl], InsĘtut Paleobiologii PAN, ul. Twarda 5 I /5 5, PL-00-8 I 8 Warszawa, Poland. DemberĘin Dash7eveg, Geological Institute, Mongolian Academy of Sciences, Ulan Bator, Mongolia. Introduction Beliajeva et al. (1974) reportedthe discovery of Early Cretaceous mammals at the Khoboor locality (referred to also sometimes as Khovboor), in the Guchin Us Soinon (County), Gobi Desert, Mongolia. -

Skeletal Variation in Extant Species Enables Systematic Identification of New Zealand's Large, Subfossil Diplodactylids
Scarsbrook et al. BMC Ecol Evo (2021) 21:67 BMC Ecology and Evolution https://doi.org/10.1186/s12862-021-01808-7 RESEARCH Open Access Skeletal variation in extant species enables systematic identifcation of New Zealand’s large, subfossil diplodactylids Lachie Scarsbrook1*, Emma Sherratt2, Rodney A. Hitchmough3 and Nicolas J. Rawlence1 Abstract New Zealand’s diplodactylid geckos exhibit high species-level diversity, largely independent of discernible osteologi- cal changes. Consequently, systematic afnities of isolated skeletal elements (fossils) are primarily determined by comparisons of size, particularly in the identifcation of Hoplodactylus duvaucelii, New Zealand’s largest extant gecko species. Here, three-dimensional geometric morphometrics of maxillae (a common fossilized element) was used to determine whether consistent shape and size diferences exist between genera, and if cryptic extinctions have occurred in subfossil ‘Hoplodactylus cf. duvaucelii’. Sampling included 13 diplodactylid species from fve genera, and 11 Holocene subfossil ‘H. cf. duvaucelii’ individuals. We found phylogenetic history was the most important predictor of maxilla morphology among extant diplodactylid genera. Size comparisons could only diferentiate Hoplodactylus from other genera, with the remaining genera exhibiting variable degrees of overlap. Six subfossils were positively identifed as H. duvaucelii, confrming their proposed Holocene distribution throughout New Zealand. Conversely, fve subfossils showed no clear afnities with any modern diplodactylid genera, implying either increased morphological diversity in mainland ‘H. cf. duvaucelii’ or the presence of at least one extinct, large, broad-toed diplodactylid species. Keywords: Diplodactylidae, Ecomorphology, Geometric morphometrics, Hoplodactylus duvaucelii, Taxonomy Background (e.g. coloration and scalation), with interspecifc skeletal New Zealand’s lizard fauna is characteristic of isolated variation rarely analysed. -
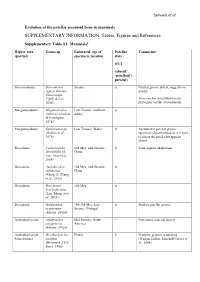
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp.