2019 DIA China Annual Meeting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2017 Sustainability Report
2017 SUSTAINABILITY REPORT CREATING A BETTER WORLD CONTENTS WHO WE ARE CREATING A BETTER WORLD 4 About this Report 10 Business Overview 24 Our Goals 6 Letter from our CEO 13 Our Products 29 Our Planet 8 About Medidata OUR APPROACH GRI INDEX 14 Stalkholders 30-32 GRI Index 16 Materials References: PEOPLE FIRST Priority Issue: An issue deemed material to the company through our sustainability issue prioritization process. 18 Ethics Global Reporting Initiative (GRI): The GRI Sustainability Reporting Standards are the first and 20 Our People most widely adopted global standards for sustainability reporting. 22 Diversity Matters UN Global Compact: The UNGC is a voluntary initiative based on CEO commitments to implement universal sustainability principles and to understand partnerships in support of UN Goals. Sustainable Development Goals: The SDGs are a universal call to action to end poverty, protect the planet, and ensure that all people enjoy peace and prosperity. 2 2017 MEDIDATA SUSTAINABILITY REPORT 2017 MEDIDATA SUSTAINABILITY REPORT 3 CREATING A CREATING A WHO WE ARE OUR APPROACH PEOPLE FIRST BETTER WORLD GRI INDEX WHO WE ARE OUR APPROACH PEOPLE FIRST BETTER WORLD GRI INDEX About this Report Letter from our CEO About Medidata Business Overview Our Products About this Report Letter from our CEO About Medidata Business Overview Our Products ABOUT THIS REPORT As we reflect on the last 18 years, the spirit of No information contained on our website is innovation—one that has truly paved the way intended to be included as part of, or incorporated for better solutions, higher quality treatments, by reference into, this Annual Report on Form and quicker go-to-market times—remains our 10-k. -

Name of Recognized Medical Schools (Foreign)
1 Name of Recognized Medical Schools (Foreign) Expired AUSTRALIA 1 School of Medicine, Faculty of Heath, University of Tasmania, Tasmania, Australia (5 years Program) 9 Jan Main Affiliated Hospitals 2021 1. Royal H obart Hospital 2. Launceston Gen Hospital 3. NWest Region Hospital 2 Melbourne Medical School, University of Melbourne, Victoria, Australia (4 years Program) 1 Mar Main Affiliated Hospitals 2022 1. St. Vincent’s Public Hospital 2. Epworth Hospital Richmond 3. Austin Health Hospital 4. Bendigo Hospital 5. Western Health (Sunshine, Footscray & Williamstown) 6. Royal Melbourne Hospital Affiliated Hospitals 1. Pater MacCallum Cancer Centre 2. Epworth Hospital Freemasons 3. The Royal Women’s Hospital 4. Mercy Hospital for Women 5. The Northern Hospital 6. Goulburn Valley Health 7. Northeast Health 8. Royal Children’s Hospital 3 School of Medicine and Public Health, University of Newcastle, New South Wales, Australia (5 years Program) 3 May Main Affiliated Hospitals 2022 1.Gosford School 2. John Hunter Hospital Affiliated Hospitals 1. Wyong Hospital 2. Calvary Mater Hospital 3. Belmont Hospital 4. Maitland Hospital 5. Manning Base Hospital & University of Newcastle Department of Rural Health 6. Tamworth Hospital 7. Armidale Hospital 4 Faculty of Medicine, Nursing and Health Sciences, Monash University, Australia (4 and 5 years Program) 8 Nov Main Affiliated Hospitals 1. Eastern Health Clinical School: EHCS 5 Hospitals 2022 2. Southern School for Clinical Sciences: SCS 5 Hospitals 3. Central Clinical School จ ำนวน 6 Hospitals 4. School of Rural Health จ ำนวน 7 Hospital 5 Sydney School of Medicine (Sydney Medical School), Faculty of Medicine and Health, University of Sydney, Australia 12 Dec (4 years Program) 2023 2 Main Affiliated Hospitals 1. -
Curriculum Vitae
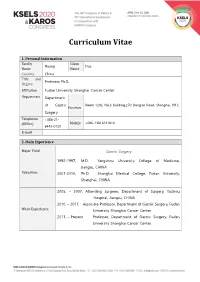
Curriculum Vitae 1. Personal Information Family Given Huang Hua Name Name Country China Title and Professor, Ph.D. Degree Affiliation Fudan University Shanghai Cancer Center Department Department of Gastric Room 1203, No.3 Building,270 Dong’an Road, Shanghai, P.R.C. Position Surgery Telephone +086-21- (Office) Mobile +086-13816151816 6443-0130 E-mail 2. Main Experience Major Field Gastric Surgery 1992-1997, M.D. Yangzhou University College of Medicine, Jiangsu, CHINA Education 2007-2010, Ph.D. Shanghai Medical College, Fudan University, Shanghai, CHINA 2005, – 2007, Attending surgeon, Department of Surgery, Taizhou Hospital, Jiangsu, CHINA 2010, – 2017, Associate Professor, Department of Gastric Surgery, Fudan Work Experience University Shanghai Cancer Center. 2017, – Present Professor, Department of Gastric Surgery, Fudan University Shanghai Cancer Center. 1. Hua Huang, XiaoFei Zhang, HaiJun Zhou, YuHua Xue, QiongZhu Dong, QingHai Ye, LunXiu Qin. Expression of osteopontin and caspase-3 and its prognostic significance in hepatocellular carcinoma patients after curative resection. Cancer Science. 2010;101(5):1314-1319. 2. HaiJun Zhou, Hua Huang, Jiong Shi, Yue Zhao, QiongZhu Dong, HuLiang Jia,YinKun Liu, QingHai Ye,huichuan sun, Xiaodong zhu, LiYun Fu, Kun Guo, DongMei Gao, Jian Sun, ZhaoWei Yan, Ning Ren, Zhaoyou Tang, LunXiu Qin. The Prognostic Value of IL-2 and IL-15 in Peritumoral Hepatic Tissues for Hepatitis B-related Hepatocellular Carcinoma Patients after Curative Resection. Gut. 2010 Dec;59(12):1699-708. (co-first author). 3. Hua Huang, Wei Wang, Zhong Chen, Jie-Jie Jin, Zi-Wen Long, Hong Cai, Xiao-Wen Liu, Ye Zhou and Ya-Nong Wang. Prognostic factors and survival Research Interests in patients with gastric stump cancer. -

Semi-Annual Market Review
Semi-Annual Market Review HEALTH IT & HEALTH INFORMATION SERVICES JULY 2019 www.hgp.com TABLE OF CONTENTS 1 Health IT Executive Summary 3 2 Health IT Market Trends 6 3 HIT M&A (Including Buyout) 9 4 Health IT Capital Raises (Non-Buyout) 14 5 Healthcare Capital Markets 15 6 Macroeconomics 19 7 Health IT Headlines 21 8 About Healthcare Growth Partners 24 9 HGP Transaction Experience 25 10 Appendix A – M&A Highlights 28 11 Appendix B – Buyout Highlights 31 12 Appendix C – Investment Highlights 34 Copyright© 2019 Healthcare Growth Partners 2 HEALTH IT EXECUTIVE SUMMARY 1 An Accumulating Backlog of Disciplined Sellers Let’s chat about fireside chats. The term first used to describe a series of evening radio addresses given by U.S. President Franklin D. Roosevelt during the Great Depression and World War II is now investment banker speak for “soft launches” of sell-side and capital raise transactions. Every company has a price, and given a market of healthy valuations, more companies are testing the waters to find out whether they can achieve that price. That process now looks a little more informal, or how you might envision a fireside chat. Price (or valuation) discovery for a company can range from a single conversation with an individual buyer to a full-blown auction with hundreds of buyers and everything in between, including a fireside chat. Given the increasing share of informal conversations, the reality is that more companies are for sale than meets the eye. While the healthy valuations publicized and press-released are encouraging more and more companies to price shop, there is a simultaneous statistical phenomenon in perceived valuations that often goes unmentioned: survivorship bias. -

20180115-CHN-Eng.Pdf (1.114Mb)
Meeting Report GO WHO CHINA: WORKING IN WHO 15 January 2018 Beijing, People's Republic of China GO WHO China: Working in WHO 15 January 2018 Beijing, People's Republic of China WORLD HEALTH ORGANIZATION REGIONAL OFFICE FOR THE WESTERN PACIFIC English only MEETING REPORT GO WHO CHINA: WORKING IN WHO Convened by: WORLD HEALTH ORGANIZATION REGIONAL OFFICE FOR THE WESTERN PACIFIC Beijing, People’s Republic of China 15 January 2018 Not for sale Printed and distributed by: World Health Organization Regional Office for the Western Pacific Manila, Philippines February 2018 NOTE The views expressed in this report are those of the participants of the Go WHO Workshops to Improve Geographical Representation of WHO Staff and do not necessarily reflect the policies of the conveners. This report has been prepared by the World Health Organization Regional Office for the Western Pacific for Member States in the Region and for those who participated in the Go WHO Workshops to Improve Geographical Representation of WHO Staff in Beijing, People’s Republic of China on 15 January 2018. CONTENTS SUMMARY ............................................................................................................................................ 1 1. INTRODUCTION .............................................................................................................................. 2 1.1 Background ................................................................................................................................... 2 1.2 Workshop objectives .................................................................................................................... -

Prof. Weiliu Qiu: Be a “Bowing” Doctor
Meet the Professor Page 1 of 9 Prof. Weiliu Qiu: be a “bowing” doctor Received: 13 July 2019; Accepted: 01 August 2019; Published: 09 August 2019. doi: 10.21037/fomm.2019.08.02 View this article at: http://dx.doi.org/10.21037/fomm.2019.08.02 Upon seeing his “savior” in the ward, the old man would get so excited that he would get up from bed outright. “Slowly, slowly!” academician Weiliu Qiu hurriedly held up the old man, bent down and picked up the slippers on the ground to put on his feet. The old man was completely bewildered at the sight of a 70-year-old academician putting on shoes for him. “How can this be done? How can this be done?” This small moment happened more than a decade ago, but is still heartwarming to this day. Weiliu Qiu (Figure 1) is one of the founders and pioneers of oral and maxillofacial surgery in China, and the first member of the Chinese Academy of Engineering in the field of stomatology. He was the former president of Shanghai Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University and director of the university’s College Figure 1 Academician Weiliu Qiu. of Stomatology. During his 60-year career, he has set a number of records in the field of oral and maxillofacial surgery, and cured numerous patients. surgery, and oral and maxillofacial repair/reconstruction When reflecting on his life, Prof. Qiu says in an surgery in China. He has won three National Invention emotional tone, “patients are teachers of doctors, and they bring Awards and Science and Technology Progress Awards, and us more than what we have given to them. -

Chinese Guidelines for the Diagnosis and Treatment of Hospital- Acquired Pneumonia and Ventilator-Associated Pneumonia in Adults (2018 Edition)
2616 Guideline Chinese guidelines for the diagnosis and treatment of hospital- acquired pneumonia and ventilator-associated pneumonia in adults (2018 Edition) Yi Shi1#, Yi Huang2#, Tian-Tuo Zhang3#, Bin Cao4#, Hui Wang5#, Chao Zhuo6#, Feng Ye6#, Xin Su1#, Hong Fan7#, Jin-Fu Xu8#, Jing Zhang9#, Guo-Xiang Lai10#, Dan-Yang She11#, Xiang-Yan Zhang12, Bei He13, Li-Xian He9, You-Ning Liu14, Jie-Ming Qu15; on behalf of Infection Study Group of Chinese Thoracic Society, Chinese Medical Association 1Department of Pulmonary and Critical Care Medicine, Nanjing Jinling Hospital, Nanjing University, School of Medicine, Nanjing 210002, China; 2Department of Pulmonary and Critical Care Medicine, Shanghai Changhai hospital, Navy Medical University, Shanghai 200433, China; 3Department of Pulmonary and Critical Care Medicine, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou 510630, China; 4Department of Pulmonary and Critical Care Medicine, China-Japan Friendship Hospital, Capital Medical University, Beijing 100029, China; 5Department of Clinical Laboratory Medicine, Peking University People’s Hospital, Beijing 100044, China; 6State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou Medical University, Guangzhou 510120, China; 7Department of Pulmonary and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu 610041, China; 8Department of Pulmonary and Critical -

Author Correction: KRAB-Type Zinc-Finger Proteins PITA and PISA
www.nature.com/cr www.cell-research.com CORRECTION Author Correction: KRAB-type zinc-finger proteins PITA and PISA specifically regulate p53-dependent glycolysis and mitochondrial respiration Shan Wang1,2,3, Zhiqiang Peng1,2, Siying Wang4, Lihua Yang4, Yuhan Chen1,2, Xue Kong4, Shanshan Song1,2, Pei Pei3, Chunyan Tian1,2, Hui Yan5, Peipei Ding6, Weiguo Hu6, Cui Hua Liu 7, Xin Zhang8, Fuchu He1,2 and Lingqiang Zhang1,2,9 Cell Research (2018) 28:605; https://doi.org/10.1038/s41422-018-0026-6 Correction to: Cell Research (2018) 0:1-21. https://doi.org/10.1038/ s41422-018-0008-8; published online 21 February 2018. We apologize for an error that we just found in the paper published online on 21 February 2018. The 1st row/1st column of Fig. 8g (PITA Normal) was inadvertently duplicated from 2nd row/ 1st column of Fig. 8i (PISA Adjacent colonic mucosa). The 2nd row/ 1st column of Fig. 8g (PISA Normal) was inadvertently duplicated from 2nd row/2nd column of Fig. 8g (PISA Distal colonic mucosa). A corrected version of Fig. 8g is provided below. No conclusion was affected by this error, but we apologize for not detecting it Fig. 8 PITA and PISA promote tumorigenesis. g Representative before publication and any inconvenience caused. images of immunohistochemical staining for PITA and PISA are shown. Scale bar, 50 μm 1State Key Laboratory of Proteomics, Beijing Proteome Research Center, National Center of Protein Sciences (Beijing), Beijing Institute of Lifeomics, Beijing, China; 2Department of Genomics and Proteomics, Beijing Institute of Radiation -

4Th DIA China Annual Meeting
ײࣷනٷ ˖࿋ڇࢇӸ 35(/,0,1$5< 352*5$0 ዐࡔᅅᄱࡔाୁዐ႐ ',$ ዐࡔࣷ ຺াᄱ႑တၹࣷڼ ႎظዐࡔᇑ๘হüüࢇፕᇑ WK',$&KLQD$QQXDO0HHWLQJ &ROODERUDWLRQ ,QQRYDWLRQLQ&KLQD ዐࡔฉ࡛ࡔाࣷᅱዐ႐ 6KDQJKDL,QWHUQDWLRQDO&RQYHQWLRQ&HQWHU&KLQD ሆනȍȍጆ༶ಢჟ ሆනȍȍࣷᅱԒߢĂ༪ஃĂቛબࢅஃ࿔ՄԒ 0D\3UHFRQIHUHQFH:RUNVKRSV 0D\&RQIHUHQFH([KLELWLRQDQG3RVWHUV WWW.DIACHINA.ORG John J. HU, PhD Vice President and General Manager, United States Pharmacopeia-China Welcome to 4th DIA China Annual Meeting in Shanghai! This year’s theme, “Collaboration and Innovation in China”, speaks to the fact that future pharmaceutical innovation is about collaboration, and China is an important member of the global innovation community that will contribute to this partnership. Thanks to the outstanding hard work of the program committee and DIA-China staff, we have added exciting new events to this year’s program. We are delighted and hon- ored to have Dr. Yin Li, the commissioner of SFDA, and two distinguished international thought leaders, Dr. David Ho and Dr. Alberto Grignolo as keynote speakers. For the first time, SFDA’s Center for Drug Evaluation (CDE), has agreed to conduct a town hall meeting that will allow meeting participants have an open and direct dialog with CDE. Working with China’s SFDA, US FDA and US Department of Commerce, we have also added two new API regulatory workshops at the preconference session. In addition, we are fortunate to be able to invite Japan’s PMDA to provide a special session focus- ing on recent regulatory policy development in the world’s second largest pharmaceu- tical market. New to this year’s program are dedicated tracks on biological and vac- cine development, CRO collaborations and China’s various high-tech parks that offer government incentives for pharmaceutical R&D activities. -

Enhanced Activities of Blood Thiamine Diphosphatase and Monophosphatase in Alzheimer’S Disease
RESEARCH ARTICLE Enhanced Activities of Blood Thiamine Diphosphatase and Monophosphatase in Alzheimer's Disease Xiaoli Pan1,2☯, Shaoming Sang1,2☯, Guoqiang Fei1, Lirong Jin1, Huimin Liu2, Zhiliang Wang3, Hui Wang3, Chunjiu Zhong1,2* 1 Department of Neurology, Zhongshan Hospital & Shanghai Medical College, Fudan University, Shanghai, China, 2 State Key Laboratory of Medical Neurobiology, Institutes of Brain Science & Collaborative Innovation Center for Brain Science, Fudan University, Shanghai, China, 3 Regional Health Service Center of a11111 Xujiahui, Xuhui District, Shanghai, China ☯ These authors contributed equally to this work. * [email protected] Abstract OPEN ACCESS Citation: Pan X, Sang S, Fei G, Jin L, Liu H, Wang Z, et al. (2017) Enhanced Activities of Blood Background Thiamine Diphosphatase and Monophosphatase in Thiamine metabolites and activities of thiamine-dependent enzymes are impaired in Alzhei- Alzheimer's Disease. PLoS ONE 12(1): e0167273. mer's disease (AD). doi:10.1371/journal.pone.0167273 Editor: Marie-Claude Potier, Institut du cerveau et de la moelle epiniere, FRANCE Objective Received: April 17, 2016 To clarify the mechanism for the reduction of thiamine diphosphate (TDP), an active form of Accepted: November 12, 2016 thiamine and critical coenzyme of glucose metabolism, in AD. Published: January 6, 2017 Copyright: © 2017 Pan et al. This is an open access Methods article distributed under the terms of the Creative Forty-five AD patients clinically diagnosed and 38 age- and gender-matched control sub- Commons Attribution License, which permits unrestricted use, distribution, and reproduction in jects without dementia were voluntarily recruited. The contents of blood TDP, thiamine any medium, provided the original author and monophosphate (TMP), and thiamine, as well as the activities of thiamine diphosphatase source are credited. -

A Hydrostatic Pressure-Driven Passive Micropump Enhanced with Siphon-Based Autofill Function
Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is © The Royal Society of Chemistry 2018 A hydrostatic pressure-driven passive micropump enhanced with siphon-based autofill function Xiaolin Wang‡abc, Da Zhao‡d, Duc T.T. Phane, Jingquan Liuabc, Xiang Chenabc, Bin Yangabc, Christopher C.W. Hughesde, Weijia Zhang*fg, Abraham P. Lee*dh a Department of Micro/Nano Electronics, School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, Shanghai 200240, P. R. China b National Key Laboratory of Science and Technology on Micro/Nano Fabrication, Department of Micro/Nano Electronics, School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, Shanghai 200240, P. R. China c Key Laboratory for Thin Film and Microfabrication Technology (Ministry of Education), Department of Micro/Nano Electronics, School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, Shanghai 200240, P. R. China d Department of Biomedical Engineering, University of California, Irvine, CA 92697, USA. E-mail: [email protected]; Fax: +1 (949) 824 1727;Tel: +1 (949) 824 9691 e Department of Molecular Biology & Biochemistry, University of California, Irvine, CA 92697, USA f The Fifth People’s Hospital of Shanghai and Institutes of Biomedical Sciences, Shanghai Medical College, Fudan University, Shanghai 200032, China. E-mail: [email protected]; Tel: +86 (021) 5423 7385 g State Key Laboratory of Molecular Engineering of Polymers, Fudan University, Shanghai 200433, China h Department of Mechanical and Aerospace Engineering, University of California, Irvine, CA 92697, USA ‡ These authors contributed equally to this work * These authors contributed equally as senior authors and correspondents to this work Corresponding authors: Weijia Zhang Email: [email protected]; Tel: +86 (021) 5423-7385. -

COVID-19 and Clinical Trials: the Medidata Perspective Release 7.0
MAYJULY 18, 13, 2020 2020 COVID-19 and Clinical Trials: The Medidata Perspective Release 7.0 Copyright 2020 Medidata Solutions, Inc., a Dassault Systèmes company JULY 13, 2020 2 COVID-19 AND CLINICAL TRIALS: THE MEDIDATA PERSPECTIVE Table of Contents What’s New/What’s Significantly Updated in Release 7.0 3 Insights to Ongoing Data Capture in Clinical Trials 3 Regulatory Response 5 Impact to Medidata Customers, Patients and Trials 6 The Race for a Vaccine 7 Medidata Solutions to Assist Sponsors/Partners, Patients and Trials 10 Details on New and Adapted Medidata Solutions 11 Summary 18 Copyright 2020 Medidata Solutions, Inc., a Dassault Systèmes company JULY 13, 2020 3 COVID-19 AND CLINICAL TRIALS: THE MEDIDATA PERSPECTIVE What’s New/What’s Significantly Updated in Release 7.0 ā New: Metrics on new patients entering trials by country/region and therapeutic area by month ā Updated: Regulatory Response ā Updated:The Race for a Vaccine ā Updated: Summary table of the current vaccine clinical trials for COVID-19 ā Updated: Graphical Representation of COVID-19 Vaccination Trials ā Updated: Summary Insights to Ongoing Data Capture in Clinical Trials Medidata is continuously monitoring the global impact of COVID-19 on clinical trials. Our first data and insights impact report was released on March 23, with subsequent releases on April 3, April 17, May 4, May 18, June 15 and now July 13. At the beginning of the pandemic, we were looking at year-over-year changes to understand and grasp the magnitude of COVID-19 on the impact on clinical trials in terms of trial activity, across geographies and therapeutic areas (TAs).