EFSA Compendium Botanicals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Essential Oils
-Essential Oils- Essential Oils エッセンシャルオイル An essential oil is a natural, 100% pure oil extracted by distillation or another method from various sources such as aromatic substances produced in the flowers, buds, leaves, peel, bark, roots, or seeds of plants, or organs of certain animals that secrete aromatic components. Essential oils are used in aromatherapy, a natural remedy to cure psychological stress and poor physical conditions, and are also used as food flavorings to be added to beverages, confectionery and other processed foods, and as cosmetic fragrances for perfume products or toiletries. Essential oils have long been used for scenting purposes such as food flavorings and cosmetic fragrances, but the glowing popularity of aromatherapy in recent years has resulted in increased uses of essential oils for aromatherapy. Scope of coverage Item Definition HS Code Citrus essential oils Of orange 3301.12.000 Of lemon 3301.13.000 Other (bergamot, other) 3301.19 Non-citrus essential oils Other peppermint (Mentha piperita) 3301.24.000 Of other mints 3301.25 Other 3301.29. Resinoid - 3301.30.000 Other - 3301.90.000 Note: Resinoid refers to liquid, semisolid, or solid substances extracted from plant resins or other sources with the use of a hydrocarbon solvent. 1. Points To Note in Exports to and Sales in Japan (1) Import Regulation and Procedures Importing essential oils may be subject to regulations under the Foreign Exchange and Foreign Trade Act, based on the CITES[Convention on International Trade in Endangered Species of Wild Fauna and Flora]. Different procedures may also be required according to uses of essential oils. -

The Following Carcinogenic Essential Oils Should Not Be Used In
Aromatherapy Undiluted- Safety and Ethics Copyright © Tony Burfield and Sylla Sheppard-Hanger (2005) [modified from a previous article “A Brief Safety Guidance on Essential Oils” written for IFA, Sept 2004]. Intro In the last 20 years aromatherapy has spread its influence to the household, toiletries and personal care areas: consumer products claiming to relax or invigorate our psyche’s have invaded our bathrooms, kitchen and living room areas. The numbers of therapists using essential oils in Europe and the USA has grown from a handful in the early 1980’s to thousands now worldwide. We have had time to add to our bank of knowledge on essential oils from reflecting on many decades of aromatherapeutic development and history, the collection of anecdotal information from practicing therapists, as well as from clinical & scientific investigations. We have also had enough time to consider the risks in employing essential oils in therapy. In the last twenty years, many more people have had accidents, been ‘burnt’, developed rashes, become allergic, and become sensitized to our beloved tools. Why is this? In this paper, we hope to shed light on this issue, clarify current safety findings, and discuss how Aromatherapists and those in the aromatherapy trade (suppliers, spas, etc.) can interpret this data for continued safe practice. After a refresher on current safety issues including carcinogenic and toxic oils, irritant and photo-toxic oils, we will look at allergens, oils without formal testing, pregnancy issues and medication interactions. We will address the increasing numbers of cases of sensitization and the effect of diluting essential oils. -

Hylotelephium National Protocol: NP/HYL/1.Rev ______Botanical Taxon: Hylotelephium Telephium (L.) H
Hylotelephium National protocol: NP/HYL/1.rev ____________________________________________________________________ Botanical taxon: Hylotelephium telephium (L.) H. Ohba x H. spectabile (Boreau) H. Ohba (syn. Sedum telephium L. x S. spectabile Boreau) Common Name (when known): Sedum Date of preparation of NP: 28-08-2012 Date of revision of NP: 01-01-2020 NP revised by: W.A. Wietsma Sample to be examined: VEGETATIVE Number of growing cycles: 1 year Closing date for applications: 1/12 Submission date/period: 1/4 - 30/4 Seed/Plant Quantity: 24 young plants able to show all their characteristics during the first year of examination Special conditions sample: None Test station address: Naktuinbouw, Sotaweg 22, 2371 AA, Roelofarendsveen Name: Team Support Variety Testing Department E-mail: [email protected] List of grouping characteristics: NO, (if yes put as annex) Minimum number of plants in trial: Vegetative: 20 Seed: not appl. Minimum number of plants observed by measuring or counting: Vegetative: 1 Seed: not appl. Give description of when observations on the flower should take place: At full flowering Give description of when/where observations on the leaf should take place: At full flowering Give description of when/where the other observations should take place: At full flowering Test will take place: OUTDOOR Uniformity: A population standard of 1% with an acceptance probability of at least 95%. Number of Off-types allowed: one off-type allowed in a sample size of 24 Table of characteristics: PRESENT (see annex) (if present, please annex the table of characteristics and explanations) Literature: PRESENT (when present, please annex to this document) Page 1 of 3 Table of characteristics Hylotelephium 1. -

The Cyanogenic Polymorphism in Trifolium Repens L
Heredity66 (1991) 105—115 Received 16 May 1990 Genetical Society of Great Britain The cyanogenic polymorphism in Trifolium repens L. (white clover) M. A. HUGHES Department of Biochemistry and Genetics, The Medical School, The University, Newcastle upon Tyne NE2 4HH Thecyanogenic polymorphism in white clover is controlled by alleles of two independently segregating loci. Biochemical studies have shown that non-functional alleles of the Ac locus, which controls the level of cyanoglucoside produced in leaf tissue, result in the loss of several steps in the biosynthetic pathway. Alleles of the Li locus control the synthesis of the hydrolytic enzyme, linamarase, which is responsible for HCN release following tissue damage. Studies on the selective forces and the distribution of the cyanogenic morphs of white clover are discussed in relation to the quantitative variation in cyanogenesis revealed by biochemical studies. Molecular studies reveal considerable restriction fragment length polymorphism for linamarase homologous genes. Keywords:cyanogenesis,polymorphism, Trifolium repen, white clover. genetics to plant taxomony. This review discusses the Introduction extensive and diverse ecological genetic studies in rela- Theterm cyanogenesis describes the release of hydro- tion to the more recent biochemical and molecular cyanic acid (HCN), which occurs when the tissues of studies of cyanogenesis in white clover. some plant species are damaged. The first report of cyanogenesis in Trifolium repens (white clover) was by Mirande (1912) and this was shortly followed by a Biochemistry paper which demonstrated that the species was poly- Theproduction of HCN by higher plants depends morphic for the character, with both cyanogenic and upon the co-occurrence of a cyanogenic glycoside and acyanogenic plants occurring in the same population catabolic enzymes. -

Sedum Society Newsletter(130) Pp
Open Research Online The Open University’s repository of research publications and other research outputs Kalanchoe arborescens - a Madagascan giant Journal Item How to cite: Walker, Colin (2019). Kalanchoe arborescens - a Madagascan giant. Sedum Society Newsletter(130) pp. 81–84. For guidance on citations see FAQs. c [not recorded] https://creativecommons.org/licenses/by-nc-nd/4.0/ Version: Version of Record Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk NUMBER 130 SEDUM SOCIETY NEWSLETTER JULY 2019 FRONT COVER Roy Mottram kindly supplied: “The Diet” copy of this Japanese herbal which is sharp and crisp (see page 97). “I counted the plates, and this copy is complete with 200 plates, in 8 parts, bound here in 2 vols. I checked for another Sedum but none are Established April 1987, now ending our present, so Maximowicz was basing his 32nd year. S. kagamontanum on this same plate, Subscriptions run from October to the following September. Anyone requesting translating the location as Mt. Kaga and to join after June, unless there is a special citing t.40 incorrectly. The "t.43" plate request, will receive his or her first number is also wrong. It is actually t.33 of Newsletter in October. If you do not the whole work, or Vol.2 t.8. The book is receive your copy by the 10th of April, July or October, or the 15th January, then bound back to front [by Western standards] please write to the editor: Ray as in all Japanese books of the day.” RM. -

Compendium of Botanicals Reported to Contain Naturally Occuring Substances of Possible Concern for Human Health When Used in Food and Food Supplements
View metadata,Downloaded citation and from similar orbit.dtu.dk papers on:at core.ac.uk Dec 20, 2017 brought to you by CORE provided by Online Research Database In Technology Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements EFSA authors; Pilegaard, Kirsten Link to article, DOI: 10.2903/j.efsa.2012.2663 Publication date: 2012 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): EFSA authors (2012). Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements. Parma, Italy: Europen Food Safety Authority. (The EFSA Journal; No. 2663, Vol. 10(5)). DOI: 10.2903/j.efsa.2012.2663 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. EFSA Journal 2012;10(5):2663 SCIENTIFIC REPORT OF EFSA Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements1 European Food Safety Authority2, 3 European Food Safety Authority (EFSA), Parma, Italy ABSTRACT In April 2009, EFSA published on its website a Compendium of botanicals reported to contain toxic, addictive, psychotropic or other substances of concern. -

CERTIFICATE of ANALYSIS (COA)
CERTIFICATE of ANALYSIS (COA) COMMON NAME Ravensara - Wild LATIN NAME Ravensara aromatica COUNTRY OF ORIGIN Madagascar CULTIVATION METHOD Wild Grown TYPE Essential Oil EXTRACTION METHOD Steam Distilled PLANT PART Leaves USE Aromatherapy, Natural Perfumery SKU 835 LOT # 16 MANUFACTURING DATE June 2016 BEST BY DATE June 2019 SPECIFICATIONS (Range) SPECIFIC GRAVITY @20˚C na 0.894 REFRACTIVE INDEX @20˚C na 1.432 OPTICAL ROTATION @20˚C 0 to +10˚ na PHYSICAL APPEARANCE Transparent liquid Conforms COLOR Colorless Conforms ODOR Fatty, fruity odor Conforms SOLUBILITY Soluble in alcohol and fixed oils SPECIAL USE INSTRUCTIONS Dilute before use. PRIMARY CONSTITUENTS Limonene, Trans-Caryophyllene, Sabinene, Linalool, Methyleugenol COMPONENTS % RANGE % COMPONENTS % RANGE % ALPHA-THUJENE na 0.69 ALPHA-COPAENE na 1.55 ALPHA-PINENE na 3.71 BETA-CUBEBENE na 0.35 CAMPHENE na 0.54 BETA-ELEMENE na 0.55 SABINENE na 8.44 METHYLEUGENOL* <10 7.17 BETA-PINENE na 2.51 TRANS-CARYOPHYLLENE na 10.66 MYRCENE <=7.3 2.34 TRANS-ALPHA-BERGAMOTENE na 0.8 ALPHA-PHELLANDRENE na 1.54 ALPHA-HUMULENE na 1.43 DELTA-3-CARENE na 3.76 ALLOAROMADENDRENE na 0.19 ALPHA-TERPINENE na 1.23 TRANS-CADINA-1(6),4-DIENE na 0.25 META-CYMENE na 0.07 GERMACRENE D na 2.91 PARA-CYMENE na 0.49 BETA-SELINENE na 0.13 LIMONENE* 13.9-22.5 19.86 METHYL ISOEUGENOL na 0.27 BETA-PHELLANDRENE na 1.08 BICYCLOGERMACRENE na 0.42 1,8-CINEOLE na 0.86 ALPHA-MUUROLENE na 0.17 Z-BETA-OCIMENE na 0.71 BETA-BISABOLENE na 0.49 E-BETA-OCIMENE na 0.09 GAMMA-CADINENE na 0.14 GAMMA-TERPINENE na 2.04 DELTA-CADINENE na 0.89 -

NJ Native Plants - USDA
NJ Native Plants - USDA Scientific Name Common Name N/I Family Category National Wetland Indicator Status Thermopsis villosa Aaron's rod N Fabaceae Dicot Rubus depavitus Aberdeen dewberry N Rosaceae Dicot Artemisia absinthium absinthium I Asteraceae Dicot Aplectrum hyemale Adam and Eve N Orchidaceae Monocot FAC-, FACW Yucca filamentosa Adam's needle N Agavaceae Monocot Gentianella quinquefolia agueweed N Gentianaceae Dicot FAC, FACW- Rhamnus alnifolia alderleaf buckthorn N Rhamnaceae Dicot FACU, OBL Medicago sativa alfalfa I Fabaceae Dicot Ranunculus cymbalaria alkali buttercup N Ranunculaceae Dicot OBL Rubus allegheniensis Allegheny blackberry N Rosaceae Dicot UPL, FACW Hieracium paniculatum Allegheny hawkweed N Asteraceae Dicot Mimulus ringens Allegheny monkeyflower N Scrophulariaceae Dicot OBL Ranunculus allegheniensis Allegheny Mountain buttercup N Ranunculaceae Dicot FACU, FAC Prunus alleghaniensis Allegheny plum N Rosaceae Dicot UPL, NI Amelanchier laevis Allegheny serviceberry N Rosaceae Dicot Hylotelephium telephioides Allegheny stonecrop N Crassulaceae Dicot Adlumia fungosa allegheny vine N Fumariaceae Dicot Centaurea transalpina alpine knapweed N Asteraceae Dicot Potamogeton alpinus alpine pondweed N Potamogetonaceae Monocot OBL Viola labradorica alpine violet N Violaceae Dicot FAC Trifolium hybridum alsike clover I Fabaceae Dicot FACU-, FAC Cornus alternifolia alternateleaf dogwood N Cornaceae Dicot Strophostyles helvola amberique-bean N Fabaceae Dicot Puccinellia americana American alkaligrass N Poaceae Monocot Heuchera americana -
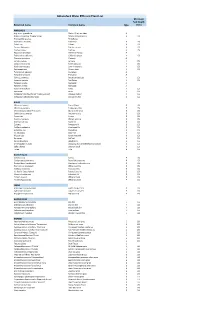
Abbotsford Water Efficient Plant List Minimum Soil Depth Botanical Name Common Name Type (Mm)
Abbotsford Water Efficient Plant List Minimum Soil Depth Botanical name Common name type (mm) ANNUALS Argemone grandifolia Statice/Sea Lavendar A Begonia x hybrida 'Dragon Wings' Dragon Wing begonia A 150 Bracteantha species Strawflower Calendula officinalis Calendula A 150 Coleus ssp. Coleus A 150 Cosmos bipinnatus Garden cosmos A 150 Cuphea llavea Cuphea A 150 Dyssocua tenuiloba Dahlberg Daisey A Eschscholzia californica California poppy A 150 Gazania spendens Gazania A Lantana camara Lantana A 150 Lobularia maritima Sweet alyssum A 150 Nigella damascena Love-in-the-mist A 150 Oesteopermum African daisy A 150 Pelargonium species Geranium A Portulaca oleracea Portulaca A Salvia guaranitica Anise-scented sage A 150 Scaevola aemula Fan flower A 150 Targetes erecta Marogold A Targetes erecta Marogold A Viola x wittrockiana Pansy A 150 Zinnia ssp. Zinnia A 150 Verbascum bombyciferum 'Arctic Summer' Broussa mullein A 150 Verbascum phlomoides 'Spica' Orange mullein A 150 BULBS Allium christophii Star of Persia B 150 Allium karataviense Turkestan onion B 150 Chionodoxa forbesii 'Pink Giant' Glory of the snow B 150 Colchicum autumnale Autumn crocus B 150 Crocus ssp. Crocus B 150 Eranthis hyemalis Winter aconite B 150 Erythronium ssp. Fawn lily B 150 Eucomis Pineapple lily B 150 Fritillaria meleagris Checkered lily B 150 Galanthus ssp. Snowdrop B 150 Iris reticulata Dwarf iris B 150 Muscari ssp. Grape hyacinth B 150 Narcissus Daffodil B 150 Nerine bowdenii Bowden lily B 150 Ornithogalum nutans Drooping Star of Bethlehem/Silverbells B 150 Scilla -

Rapid Detection Method to Quantify Linamarin Content in Cassava Dinara S
essing oc & pr B o io i t B e Gunasekera et al, J Bioprocess Biotech 2018, 8:6 f c h o n l Journal of Bioprocessing & DOI: 10.4172/2155-9821.1000342 i a q n u r e u s o J Biotechniques ISSN: 2155-9821 Research Article Open Access Rapid Detection Method to Quantify Linamarin Content in Cassava Dinara S. Gunasekera*, Binu P. Senanayake, Ranga K. Dissanayake, M.A.M. Azrin, Dhanushi T. Welideniya, Anjana Delpe Acharige, K.A.U. Samanthi, W.M.U.K. Wanninayake, Madhavi de Silva, Achini Eliyapura, Veranja Karunaratne and G.A.J Amaratunga. Sri Lanka Institute of Nanotechnology, Homagama, Sri Lanka *Corresponding author: Dinara S Gunasekera, Senior Research Scientist, Sri Lanka Institute of Nanotechnology, Homagama, Sri Lanka, Tel: +94775448584; E-mail: [email protected] Received date: November 21, 2018; Accepted date: December 12, 2018; Published date: December 20, 2018 Copyright: © 2018 Gunasekera DS et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract There are a number of natural remedies against cancer. Cassava (Manihot esculenta Crantz) has been proven to be a natural remedy against cancer due to the cyanogenic compounds it contains such as linamarin. A rapid and simple liquid chromatography-mass spectroscopy (LC-MS) method was developed to identify and quantify linamarin in Cassava. The method was developed using various Cassava (Manihot esculenta Crantz) extracts. Developed application is not limited to Cassava, but can be extended to other types of linamarin containing plant materials as well. -

Gc/Ms Batch Number: R10102
GC/MS BATCH NUMBER: R10102 ESSENTIAL OIL: RAVENSARA BOTANICAL NAME: RAVENSARA AROMATICA ORIGIN: MADAGASCAR KEY CONSTITUENTS PRESENT IN THIS BATCH OF % RAVENSARA OIL LIMONENE 16.7 SABINENE 15.5 GERMACRENE D 7.9 Δ3-CARENE 6.0 α-PINENE 5.6 β-CARYOPHYLLENE 4.5 MYRCENE 4.3 LINALOOL 3.5 SABINENE 3.2 α-PHELLANDRENE 2.9 TERPINEN-4-OL 2.4 METHYLEUGENOL 2.1 β-PHELLANDRENE 2.0 CAMPHENE 1.5 γ-TERPINENE 1.3 α-TERPINENE 1.3 cis-β-OCIMENE 1.1 δ-CADINENE 1.1 α-COPAENE 1.1 α-HUMULENE 1.1 α-THUJENE 1.0 1,8-CINEOLE 1.0 ESTRAGOLE (METHYL CHAVICOL) 1.0 Comments from Robert Tisserand: A good quality Ravensara leaf oil. This complex essential oil from Madagascar is not dominated by any single constituent. 510 2nd St S. Twin Falls, ID 83301 * 800-917-6577 * www.planttherapy.com facebook.com/PlantTherapy * www.planttherapy.com/blog Date : November 6, 2015 SAMPLE IDENTIFICATION Internal code : 15J30-PTH3-1-HM Customer identification : Ravensara - Madagascar - R10102 Type : Essential oil Source : Ravensara aromatica Customer : Plant Therapy ANALYSIS Method : PC-PA-001-15E06, "Analysis of the composition of a liquid essential oil by GC-FID" (in French). Identifications double-checked by GC-MS Analyst : Alexis St-Gelais, M. Sc. Analysis date : 2015-11-01 Page 1 of 6 Essential oil, Ravensara aromatica Customer Identification: Report prepared for Internal Code: 15J30-PTH3-1-HM Ravensara - Madagascar - R10102 Plant Therapy IDENTIFIED COMPOUNDS Column: BP5 Column: WAX Identification Molecular Class R.T. R.I. % % R.I. R.T. -

Isolation of Pure Cassava Linamarin As an Anti Cancer Agent
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Wits Institutional Repository on DSPACE ISOLATION OF PURE CASSAVA LINAMARIN AS AN ANTI CANCER AGENT CHRISTOPHER AVWOGHOKOGHENE, IDIBIE A Dissertation Submitted to the Faculty of Engineering and the Built Environment, University of the Witwatersrand, in Fulfillment of the requirement for the Degree of Master of Science in Engineering. Johannesburg, 2006. DECLARATION I declare that this dissertation is my own, unaided work. It is being submitted for the degree of Master of Science in the University of Witwatersrand, Johannesburg. It has not been submitted before for any degree or examination in any other University. (Signature of candidature) Day of ii ABSTRACT Cassava is a known source of linamarin, but difficulties associated with its isolation have prevented it from being exploited as a source. A batch adsorption process using activated carbon at the appropriate contact time proved successful in its isolation with ultrafiltration playing a pivotal role in the purification process. Result revealed that optimum purification was obtained with increasing amount of crude cassava extract (CCE) purified. 60g of CCE took 32 mins, 80 g, 34 mins while 100 g took 36 mins of contact time, where 1.7 g, 2.0 g and 2.5 g of purified product were obtained, respectively. The purification process in batch mode was also carried out at different temperatures ranging from 25 to 65oC. Results showed that purification increases with increase in temperature. In a bid to ascertain the moles of linamarin adsorbed per pore volume of activated carbon used, the composite isotherm was found to represent the measured adsorption data quite well.