Magnetic Domain Interactions of Fe3o4 Nanoparticles Embedded in a Sio2 Matrix Received: 25 October 2017 J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermal Fluctuations of Magnetic Nanoparticles: Fifty Years After Brown1)
THERMAL FLUCTUATIONS OF MAGNETIC NANOPARTICLES: FIFTY YEARS AFTER BROWN1) William T. Coffeya and Yuri P. Kalmykovb a Department of Electronic and Electrical Engineering, Trinity College, Dublin 2, Ireland b Laboratoire de Mathématiques et Physique (LAMPS), Université de Perpignan Via Domitia, 52, Avenue Paul Alduy, F-66860 Perpignan, France The reversal time (superparamagnetic relaxation time) of the magnetization of fine single domain ferromagnetic nanoparticles owing to thermal fluctuations plays a fundamental role in information storage, paleomagnetism, biotechnology, etc. Here a comprehensive tutorial-style review of the achievements of fifty years of development and generalizations of the seminal work of Brown [W.F. Brown, Jr., Phys. Rev., 130, 1677 (1963)] on thermal fluctuations of magnetic nanoparticles is presented. Analytical as well as numerical approaches to the estimation of the damping and temperature dependence of the reversal time based on Brown’s Fokker-Planck equation for the evolution of the magnetic moment orientations on the surface of the unit sphere are critically discussed while the most promising directions for future research are emphasized. I. INTRODUCTION A. THERMAL INSTABILITY OF MAGNETIZATION IN FINE PARTICLES B. KRAMERS ESCAPE RATE THEORY C. SUPERPARAMAGNETIC RELAXATION TIME: BROWN’S APPROACH II. BROWN’S CONTINUOUS DIFFUSION MODEL OF CLASSICAL SPINS A. BASIC EQUATIONS B. EVALUATION OF THE REVERSAL TIME OF THE MAGNETIZATION AND OTHER OBSERVABLES III. REVERSAL TIME IN SUPERPARAMAGNETS WITH AXIALLY-SYMMETRIC MAGNETOCRYSTALLINE ANISOTROPY A. FORMULATION OF THE PROBLEM B. ESTIMATION OF THE REVERSAL TIME VIA KRAMERS’ THEORY C. UNIAXIAL SUPERPARAMAGNET SUBJECTED TO A D.C. BIAS FIELD PARALLEL TO THE EASY AXIS IV. REVERSAL TIME OF THE MAGNETIZATION IN SUPERPARAMAGNETS WITH NONAXIALLY SYMMETRIC ANISOTROPY 1) Published in Applied Physics Reviews Section of the Journal of Applied Physics, 112, 121301 (2012). -

Dynamic Symmetry Loss of High-Frequency Hysteresis Loops in Single-Domain Particles with Uniaxial Anisotropy
Journal of Magnetism and Magnetic Materials 324 (2012) 466–470 Contents lists available at SciVerse ScienceDirect Journal of Magnetism and Magnetic Materials journal homepage: www.elsevier.com/locate/jmmm Dynamic symmetry loss of high-frequency hysteresis loops in single-domain particles with uniaxial anisotropy Gabriel T. Landi Instituto de Fı´sica da Universidade de Sao~ Paulo, 05314-970 Sao~ Paulo, Brazil article info abstract Article history: Understanding how magnetic materials respond to rapidly varying magnetic fields, as in dynamic Received 2 June 2011 hysteresis loops, constitutes a complex and physically interesting problem. But in order to accomplish a Available online 23 August 2011 thorough investigation, one must necessarily consider the effects of thermal fluctuations. Albeit being Keywords: present in all real systems, these are seldom included in numerical studies. The notable exceptions are Single-domain particles the Ising systems, which have been extensively studied in the past, but describe only one of the many Langevin dynamics mechanisms of magnetization reversal known to occur. In this paper we employ the Stochastic Landau– Magnetic hysteresis Lifshitz formalism to study high-frequency hysteresis loops of single-domain particles with uniaxial anisotropy at an arbitrary temperature. We show that in certain conditions the magnetic response may become predominantly out-of-phase and the loops may undergo a dynamic symmetry loss. This is found to be a direct consequence of the competing responses due to the thermal fluctuations and the gyroscopic motion of the magnetization. We have also found the magnetic behavior to be exceedingly sensitive to temperature variations, not only within the superparamagnetic–ferromagnetic transition range usually considered, but specially at even lower temperatures, where the bulk of interesting phenomena is seen to take place. -

Magnetic Switching of a Stoner-Wohlfarth Particle Subjected to a Perpendicular Bias Field
electronics Article Magnetic Switching of a Stoner-Wohlfarth Particle Subjected to a Perpendicular Bias Field Dong Xue 1,* and Weiguang Ma 2 1 Department of Physics and Astronomy, Texas Tech University, Lubbock, TX 79409-1051, USA 2 Department of Physics, Umeå University, 90187 Umeå, Sweden; [email protected] * Correspondence: [email protected]; Tel.: +1-806-834-4563 Received: 28 February 2019; Accepted: 21 March 2019; Published: 26 March 2019 Abstract: Characterized by uniaxial magnetic anisotropy, the Stoner-Wohlfarth particle experiences a change in magnetization leading to a switch in behavior when tuned by an externally applied field, which relates to the perpendicular bias component (hperp) that remains substantially small in comparison with the constant switching field (h0). The dynamics of the magnetic moment that governs the magnetic switching is studied numerically by solving the Landau-Lifshitz-Gilbert (LLG) equation using the Mathematica code without any physical approximations; the results are compared with the switching time obtained from the analytic method that intricately treats the non-trivial bias field as a perturbation. A good agreement regarding the magnetic switching time (ts) between the numerical calculation and the analytic results is found over a wide initial angle range (0.01 < q0 < 0.3), as h0 and hperp are 1.5 × K and 0.02 × K, where K represents the anisotropy constant. However, the quality of the analytic approximation starts to deteriorate slightly in contrast to the numerical approach when computing ts in terms of the field that satisfies hperp > 0.15 × K and h0 = 1.5 × K. Additionally, existence of a comparably small perpendicular bias field (hperp << h0) causes ts to decrease in a roughly exponential manner when hperp increases. -

Module 2C: Micromagnetics
Module 2C: Micromagnetics Debanjan Bhowmik Department of Electrical Engineering Indian Institute of Technology Delhi Abstract In this part of the second module (2C) we will show how the Stoner Wolfarth/ single domain mode of ferromagnetism often fails to match with experimental data. We will introduce domain walls in that context and explain the basic framework of micromagnetics, which can be used to model domain walls. Then we will discuss how an anisotropic exchange interaction, known as Dzyaloshinskii Moriya interaction, can lead to chirality of the domain walls. Then we will introduce another non-uniform magnetic structure known as skyrmion and discuss its stability, based on topological arguments. 1 1 Brown's paradox in ferromagnetic thin films exhibiting perpendicular magnetic anisotropy We study ferromagnetic thin films exhibiting Perpendicular Magnetic Anisotropy (PMA) to demonstrate the failure of the previously discussed Stoner Wolfarth/ single domain model to explain experimentally observed magnetic switching curves. PMA is a heavily sought after property in magnetic materials for memory and logic applications (we will talk about that in details in the next module). This makes our analysis in this section even more relevant. The ferromagnetic layer in the Ta/CoFeB/MgO stack, grown by room temperature sput- tering, exhibits perpendicular magnetic anisotropy with an anisotropy field Hk of around 2kG needed to align the magnetic moment in-plane (Fig. 1a). Thus if the ferromagnetic layer is considered as a giant macro-spin in the Stoner Wolfarth model an energy barrier equivalent to ∼2kG exists between the up (+z) and down state (-z) (Fig. 1b). Yet mea- surement shows that the magnet can be switched by a field, called the coercive field, as small as ∼50 G, which is 2 orders of magnitude smaller than the anisotropy field Hk, as observed in the Vibrating Sample Magnetometry measurement on the stack (Fig. -

Magnetically Multiplexed Heating of Single Domain Nanoparticles
Magnetically Multiplexed Heating of Single Domain Nanoparticles M. G. Christiansen,1) R. Chen,1) and P. Anikeeva1,a) 1Department of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts, 02139, USA Abstract: Selective hysteretic heating of multiple collocated sets of single domain magnetic nanoparticles (SDMNPs) by alternating magnetic fields (AMFs) may offer a useful tool for biomedical applications. The possibility of “magnetothermal multiplexing” has not yet been realized, in part due to prevalent use of linear response theory to model SDMNP heating in AMFs. Predictive successes of dynamic hysteresis (DH), a more generalized model for heat dissipation by SDMNPs, are observed experimentally with detailed calorimetry measurements performed at varied AMF amplitudes and frequencies. The DH model suggests that specific driving conditions play an underappreciated role in determining optimal material selection strategies for high heat dissipation. Motivated by this observation, magnetothermal multiplexing is theoretically predicted and empirically demonstrated for the first time by selecting SDMNPs with properties that suggest optimal hysteretic heat dissipation at dissimilar AMF driving conditions. This form of multiplexing could effectively create multiple channels for minimally invasive biological signaling applications. Text: Magnetic fields provide a convenient form of noninvasive electronically driven stimulus that can reach deep into the body because of the weak magnetic properties and low -

Magnetic Materials: Hysteresis
Magnetic Materials: Hysteresis Ferromagnetic and ferrimagnetic materials have non-linear initial magnetisation curves (i.e. the dotted lines in figure 7), as the changing magnetisation with applied field is due to a change in the magnetic domain structure. These materials also show hysteresis and the magnetisation does not return to zero after the application of a magnetic field. Figure 7 shows a typical hysteresis loop; the two loops represent the same data, however, the blue loop is the polarisation (J = µoM = B-µoH) and the red loop is the induction, both plotted against the applied field. Figure 7: A typical hysteresis loop for a ferro- or ferri- magnetic material. Illustrated in the first quadrant of the loop is the initial magnetisation curve (dotted line), which shows the increase in polarisation (and induction) on the application of a field to an unmagnetised sample. In the first quadrant the polarisation and applied field are both positive, i.e. they are in the same direction. The polarisation increases initially by the growth of favourably oriented domains, which will be magnetised in the easy direction of the crystal. When the polarisation can increase no further by the growth of domains, the direction of magnetisation of the domains then rotates away from the easy axis to align with the field. When all of the domains have fully aligned with the applied field saturation is reached and the polarisation can increase no further. If the field is removed the polarisation returns along the solid red line to the y-axis (i.e. H=0), and the domains will return to their easy direction of magnetisation, resulting in a decrease in polarisation. -

Basic Design and Engineering of Normal-Conducting, Iron-Dominated Electromagnets
Basic design and engineering of normal-conducting, iron-dominated electromagnets Th. Zickler CERN, Geneva, Switzerland Abstract The intention of this course is to provide guidance and tools necessary to carry out an analytical design of a simple accelerator magnet. Basic concepts and magnet types will be explained as well as important aspects which should be considered before starting the actual design phase. The central part of this course is dedicated to describing how to develop a basic magnet design. Subjects like the layout of the magnetic circuit, the excitation coils, and the cooling circuits will be discussed. A short introduction to materials for the yoke and coil construction and a brief summary about cost estimates for magnets will complete this topic. 1 Introduction The scope of these lectures is to give an overview of electromagnetic technology as used in and around particle accelerators considering normal-conducting, iron-dominated electromagnets generally restricted to direct current situations where we assume that the voltages generated by the change of flux and possible resulting eddy currents are negligible. Permanent and superconducting magnet technologies as well as special magnets like kickers and septa are not covered in this paper; they were part of dedicated special lectures. It is clear that it is difficult to give a complete and exhaustive summary of magnet design since there are many different magnet types and designs; in principle the design of a magnet is limited only by the laws of physics and the imagination of the magnet designer. Furthermore, each laboratory and each magnet designer or engineer has his own style of approaching a particular magnet design. -

DMI Interaction and Domain Evolution in Magnetic Heterostructures with Perpendicular Magnetic Anisotropy
Georgia State University ScholarWorks @ Georgia State University Physics and Astronomy Dissertations Department of Physics and Astronomy Spring 4-30-2018 DMI Interaction and Domain Evolution in Magnetic Heterostructures with Perpendicular Magnetic Anisotropy Jagodage Kasuni S. Nanayakkara Follow this and additional works at: https://scholarworks.gsu.edu/phy_astr_diss Recommended Citation Nanayakkara, Jagodage Kasuni S., "DMI Interaction and Domain Evolution in Magnetic Heterostructures with Perpendicular Magnetic Anisotropy." Dissertation, Georgia State University, 2018. https://scholarworks.gsu.edu/phy_astr_diss/102 This Dissertation is brought to you for free and open access by the Department of Physics and Astronomy at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Physics and Astronomy Dissertations by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. DMI INTERACTION AND DOMAIN EVOLUTION IN MAGNETIC HETEROSTRUCTURES WITH PERPENDICULAR MAGNETIC ANISOTROPY by JAGODAGE KASUNI NANAYAKKARA Under the Direction of Alexander Kozhanov, PhD ABSTRACT My thesis is dedicated to the study of the magnetic interactions and magnetization reversal dynamics in ferromagnetic heterostructures with perpendicular magnetic anisotropy (PMA). Two related projects will be included: 1) investigating interfacial Dzyaloshinskii-Moriya interaction (DMI) in multilayer structures; 2) controlled stripe domain growth in PMA heterostructures. Magneto Optic Kerr Effect microscopy and magnetometry techniques along with vibrating sample magnetometry were used to investigate these phenomena. The CoPt bi-layer system is a well-known PMA material system exhibiting DMI. However, films with many CoPt bi-layers are known as having zero effective DMI due to its inversion symmetry. I focused my research on CoNiPt tri-layer heterostructures with broken inversion symmetry. -

Successful Observation of Magnetic Domains at 500℃ with Spin
FOR IMMEDIATE RELEASE Successful observation of magnetic domains at 500℃ with spin-polarized scanning electron microscopy Contributing to magnetic material development and performance improvement of devices such as HDD Tokyo, September 21, 2010 --- Hitachi, Ltd. (NYSE : HIT/TSE : 6501, hereafter Hitachi) today announced the development of Spin-polarized Scanning Electron Microscopy (hereafter, spin-SEM) technology for observation of magnetic domains under high temperature conditions in a magnetic field. Using this technology, changes in the magnetic domain structure of a cobalt (Co) single crystal was visualized up to 500 degrees Celsius (C). By applying the technology developed, the temperature conditions for observing magnetic domains in a sample can be heated up to 500C when using only the heating unit, and up to 250C when used in combination with a function to apply a magnetic field of up to 1,000 Oersteds (Oe). As a result, it is now possible to fully utilize the spin-SEM feature of high-resolution magnetic domain observation while observing the effect of temperature and external magnetic field on magnetic materials. It is expected that in the future, this technology will contribute to the development of new materials for permanent magnets and performance improvements in magnetic devices such as hard disk drives (HDD), etc. Spin-SEM is scanning electron microscopy which focuses a squeezed electron beam on a sample surface and measures the spin (the smallest unit describing magnetic property) of the secondary electrons emitted from the sample to observe the magnetic domain (the region where the spin direction is the same). It has a high resolution (10nm for Hitachi instrument) compared with other magnetic domain observation instruments and can be used to analyze magnetization vector. -

Characterization and Quantification of Magnetic Remanence in Unexploded Ordnance
CHARACTERIZATION AND QUANTIFICATION OF MAGNETIC REMANENCE IN UNEXPLODED ORDNANCE by Whitney Elizabeth Goodrich A thesis submitted to the Faculty and Board of Trustees of the Colorado School of Mines in partial fulfillment of the requirements for the degree of Master of Science (Geophysics). Golden, Colorado Date _______________ Signed: ____________________ Whitney Elizabeth Goodrich Approved: __________________ Dr. Gary Olhoeft Thesis Advisor Golden, Colorado Date ______________ ___________________________ Dr. Terence K. Young Professor and Head Department of Geophysics ii ABSTRACT Under the Range Rule (1997), the Department of Defense defines unexploded ordnance (UXO) as a piece of ordnance that has been deployed, but has not exploded. This study investigated a subset of UXO including artillery shells and mortars (projectiles), but excluding landmines. UXO contaminates approximately 15 million acres of land in the United States alone. The geophysical tools most frequently used for detection are electromagnetic and magnetic methods. These methods, however, produce a “hit” for any metallic object in the ground, not just UXO. In a given survey, this results in a large number of anomalies, only a small subset of which are actually UXO. Current practice dictates that most anomalies are dug up and identified, and the UXO is exploded in place. The estimated cost to remediate existing UXO, in the United States, with current methods, is tens to hundreds of billions of dollars. Discrimination is the process in which UXO (hazardous) is differentiated from non-UXO (non-hazardous), prior to anomaly excavation. Methods to discriminate the UXO greatly reduce the cost of remediation. Remanent magnetization in UXO is one quantity that must be understood to improve discrimination methodologies in the future. -

Magnetic Forces ©Lluís Real/Age Fotostock
DO NOT EDIT--Changes must be made through "File info" LONumber=6P1_0610=CorrectionKey=NL-A LESSON 1 Magnetic Forces ©Lluís Real/age fotostock Magnets are able to attract or repel certain materials, such as iron, at a distance. By the end of this lesson . © Houghton Mifflin Harcourt • Image Credits: you will be able to describe the variables that affect the strength and direction of the magnetic force. 96 Unit 2 Electric and Magnetic Forces DO NOT EDIT--Changes must be made through "File info" LONumber=6P1_0610=CorrectionKey=NL-B Go online to view the digital version of the Hands-On Lab for this lesson and to download additional lab resources. CAN YOU EXPLAIN IT? Why do these rings seem to float without touching one another instead of falling? If you were to drop one of these rings onto a peg, you would normally expect it to fall and hit Explore another ring. Instead, when the rings get near one another, they are pushed back up and appear to float. ONLINE! 1. Most objects are not able to float in midair. What must be occurring for these rings to float instead of fall? ©Houghton Mifflin Harcourt 2. What type of force might be affecting the motion of these rings? © Houghton Mifflin Harcourt • Image Credits: © Houghton Mifflin Harcourt • Image Credits: EVIDENCE NOTEBOOK As you explore the lesson, gather evidence to help explain the behavior of the rings. Lesson 1 Magnetic Forces 97 DO NOT EDIT--Changes must be made through “File info” LONumber=6P1_0610=CorrectionKey=NL-B EXPLORATION 1 Describing Magnets and the Magnetic Force A magnet is an object that attracts, or pulls on, Magnet Interactions materials that contain iron. -
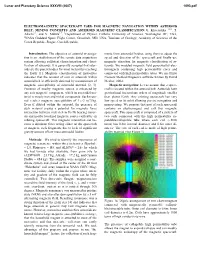
Electromagnetic Spacecraft Used for Magnetic Navigation Within Asteroid Belt, Mining Concepts and Asteroid Magnetic Classification G
Lunar and Planetary Science XXXVIII (2007) 1093.pdf ELECTROMAGNETIC SPACECRAFT USED FOR MAGNETIC NAVIGATION WITHIN ASTEROID BELT, MINING CONCEPTS AND ASTEROID MAGNETIC CLASSIFICATION G. Kletetschka 1,2,3, T. Adachi1,2, and V. Mikula1,2, 1Department of Physics, Catholic University of America, Washington DC, USA, 2NASA Goddard Space Flight Center, Greenbelt, MD, USA, 3Institute of Geology, Academy of Sciences of the Czech Republic, Prague, Czech Republic. Introduction: The objective of asteroid investiga- ments from asteroidal bodies, using them to adjust the tion is an establishment of the remote data acquisition speed and direction of the spacecraft and finally use system allowing sufficient characterization and classi- magnetic attraction for magnetic classification of as- fication of asteroids. It is generally accepted that aster- teroids. We modeled magnetic field generated by elec- oids are the parent bodies for most meteorites reaching tromagnets containing high permeability cores and the Earth [1]. Magnetic classification of meteorites connected with high permeability tubes. We use Finite indicates that the amount of iron in asteroids within Element Method Magnetics software written by David asteroid belt is efficiently detected by measurement of Meeker, 2004. magnetic susceptibility of asteroidal material [2, 3]. Magnetic navigation: Let us assume that a space- Presence of nearby magnetic source is enhanced by craft is located within the asteroid belt. Asteroids have any soft magnetic component, which in asteroidal ma- gravitational interactions orders of magnitude smaller terial is mostly iron and nickel compounds. Such mate- than planet Earth. Any orbiting spacecraft has very rial reaches magnetic susceptibility of 1 e-3 m^3/kg. low speed on its orbit allowing precise navigation and Even if diluted within the asteroid, the presence of maneuvering.