Introduction Objective Methods Results Summary References
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Product Japan : Food Processing Sector - Health and Functional Foods Company Profiles
Foreign Agricultural Service GAIN Report Global Agriculture Information Network Approved by: Date: 07/23/99 Sarah D. Hanson GAIN Report #JA9087 U.S. Embassy Market Brief - Product Japan : Food Processing Sector - Health and Functional Foods Company Profiles This report was prepared by the USDA’s Foreign Agricultural Service for U.S. exporters of food and agricultural products. This information is in the public domain and may be reprinted without permission. Use of commercial or trade names does not imply approval nor constitute endorsement by USDA/FAS. Tokyo[JA1], JA GAIN Report #JA9087 Page 1 of 24 Company Name Amway Japan Product Sector(s) Health and Functional Food Address 1-8-1, Shimo-Meguro Number Of Employees 728 Meguro-ku, Tokyo 153-8686 Number of Factories Overseas Contact Phone Number 03-5434-8484 Fax Number 03-5434-4923 Email Web Page Address www.amway.co.jp/amway_japan/ Contact Person Masura Iwata Executive Driector, External Affairs and Public Relations Sales and Net Profits Main Suppliers Year Sales (Mil. \) Net Profits 1995 177,991 22,424 1996 212,195 25,130 1997 203,361 26,638 Key Products % of Total Company Profile and Strategies Home Care Products 9 Japanese corporation of nonstore sales operator Amway (US). Housewares 30 Registered sales personnel involved in direct sales of detergents, Personal Care 34 cosmetics, kitchenware and nutritional supplements. Nutritional Supplements 23 Others 4 Main Brands Triple X (vitamin and mineral supplement), Nutri Protein, Acerola C (vitamin supplement), Salmon-Omega 3, Hon-E-Cece, Ironics, Beta Carotene A, Wheat Germ E. Main Ingredients Vitamins, protein concentrates, iron concentrates, calcium concentrates, beta caroten, wheat germ. -

FTSE Japan ESG Low Carbon Select
2 FTSE Russell Publications 19 August 2021 FTSE Japan ESG Low Carbon Select Indicative Index Weight Data as at Closing on 30 June 2021 Constituent Index weight (%) Country Constituent Index weight (%) Country Constituent Index weight (%) Country ABC-Mart 0.01 JAPAN Ebara 0.17 JAPAN JFE Holdings 0.04 JAPAN Acom 0.02 JAPAN Eisai 1.03 JAPAN JGC Corp 0.02 JAPAN Activia Properties 0.01 JAPAN Eneos Holdings 0.05 JAPAN JSR Corp 0.11 JAPAN Advance Residence Investment 0.01 JAPAN Ezaki Glico 0.01 JAPAN JTEKT 0.07 JAPAN Advantest Corp 0.53 JAPAN Fancl Corp 0.03 JAPAN Justsystems 0.01 JAPAN Aeon 0.61 JAPAN Fanuc 0.87 JAPAN Kagome 0.02 JAPAN AEON Financial Service 0.01 JAPAN Fast Retailing 3.13 JAPAN Kajima Corp 0.1 JAPAN Aeon Mall 0.01 JAPAN FP Corporation 0.04 JAPAN Kakaku.com Inc. 0.05 JAPAN AGC 0.06 JAPAN Fuji Electric 0.18 JAPAN Kaken Pharmaceutical 0.01 JAPAN Aica Kogyo 0.07 JAPAN Fuji Oil Holdings 0.01 JAPAN Kamigumi 0.01 JAPAN Ain Pharmaciez <0.005 JAPAN FUJIFILM Holdings 1.05 JAPAN Kaneka Corp 0.01 JAPAN Air Water 0.01 JAPAN Fujitsu 2.04 JAPAN Kansai Paint 0.05 JAPAN Aisin Seiki Co 0.31 JAPAN Fujitsu General 0.01 JAPAN Kao 1.38 JAPAN Ajinomoto Co 0.27 JAPAN Fukuoka Financial Group 0.01 JAPAN KDDI Corp 2.22 JAPAN Alfresa Holdings 0.01 JAPAN Fukuyama Transporting 0.01 JAPAN Keihan Holdings 0.02 JAPAN Alps Alpine 0.04 JAPAN Furukawa Electric 0.03 JAPAN Keikyu Corporation 0.02 JAPAN Amada 0.01 JAPAN Fuyo General Lease 0.08 JAPAN Keio Corp 0.04 JAPAN Amano Corp 0.01 JAPAN GLP J-REIT 0.02 JAPAN Keisei Electric Railway 0.03 JAPAN ANA Holdings 0.02 JAPAN GMO Internet 0.01 JAPAN Kenedix Office Investment Corporation 0.01 JAPAN Anritsu 0.15 JAPAN GMO Payment Gateway 0.01 JAPAN KEWPIE Corporation 0.03 JAPAN Aozora Bank 0.02 JAPAN Goldwin 0.01 JAPAN Keyence Corp 0.42 JAPAN As One 0.01 JAPAN GS Yuasa Corp 0.03 JAPAN Kikkoman 0.25 JAPAN Asahi Group Holdings 0.5 JAPAN GungHo Online Entertainment 0.01 JAPAN Kinden <0.005 JAPAN Asahi Intecc 0.01 JAPAN Gunma Bank 0.01 JAPAN Kintetsu 0.03 JAPAN Asahi Kasei Corporation 0.26 JAPAN H.U. -

Tokyo Food Technology Week 2021
Exhibit Brochure Tokyo Food Technology Week 2021 Sep. 2-3 2021 Sep. 2-3 2021 10:00~17:00 PACIFICO YOKOHAMA Exhibition Hall C [Website] https://tokyofoodtechnology.com/2021/en.pdf Organizer:EJK Japan, Ltd./ The Japan Food News Show profile The Gateway into Japan Market ! Tokyo Food Technology Week “Tokyo Food Technology Week” will be expandingly held by including “P & B JAPAN”, “Nutritious Food Ingredients EXPO”, and “food ingredients EXPO for Pre-packaged, Frozen, and Instant food”. TFTW creates the synergistic effect of 3 shows by proposing the cutting- edge technology that shows new trends in the whole food industry. LOGO nd NAME OF 2 Nutritious Food 1st food ingredients EXPO for Pre- 9th P&B JAPAN (P&B) SHOW Ingredients EXPO (NFI) packaged, Frozen, and Instant food (PFI) ORGANIZER(S) EJK Japan, Ltd. EJK Japan, Ltd./ The Japan Food News Food ingredients for bread Health functional food Food Ingredients (meat, & confectionery (flour, oil, ingredients (vitamin & sea food, vegetable, fruit, yeast, jam, honey, frozen mineral, oligo sugar, food cereal, seasoning, spice, dough, etc. ) / Food fiber, lactic acid bacteria, herb etc.) / Food additives ingredients for sandwich, sugar alcohol, protein, / Professional products for EXHBITOR burger (meat, vegetable, amino acid, enzyme, etc.) restaurant chain / Food PROFILE dairy product, sauce, etc. ) Vegetable / Herb / Honey processing technology / Coffee & tea & beverages product / Soy product / (freezing, thawing, / Kitchen equipment / POS Component analysis / sterilizer drying etc. )/ register -

Albuminuria, Expression of Nicotinamide Adenine Dinucleotide
991 Hypertens Res Vol.30 (2007) No.10 p.991-998 Original Article Albuminuria, Expression of Nicotinamide Adenine Dinucleotide Phosphate Oxidase and Monocyte Chemoattractant Protein-1 in the Renal Tubules of Hypertensive Dahl Salt-Sensitive Rats Shigemoto FUJII1), Ling ZHANG1), and Hiroaki KOSAKA1) In chronic renal diseases, experimental and human data suggest that excess albumin filtered through the glomerular capillary barrier is over-reabsorbed by proximal tubular cells, thereby activating these cells and upregulating the expression of chemokines. On the other hand, a high-salt diet has been shown to induce proteinuria in hypertensive Dahl salt-sensitive (DSS) rats, accompanied with the expression of reduced nic- otinamide adenine dinucleotide phosphate (NADPH) oxidase in the kidney. In the current study, we therefore examined albuminuria and the expressions of NADPH oxidase and monocyte chemoattractant protein-1 (MCP-1) in the renal tubular cells in hypertensive DSS rats, as well as the effects of the antioxidant N-ace- tylcysteine (NAC) on each of these parameters. DSS rats were fed a normal-salt diet (0.24% NaCl), a high- salt diet (8% NaCl), or a high-salt diet plus NAC supplementation (15 mg/mL drinking water) for 4 weeks. The high-salt diet provoked an increase in glomerular injuries accompanied with albuminuria and in urinary H2O2 and MCP-1 excretion. Immunohistochemical analysis showed the prominent expression of MCP-1 in the dilated tubular cells, where the NADPH oxidase subunit p47phox was also expressed. The current results suggest that albuminuria caused expression of NADPH oxidase and MCP-1 in the dilated renal tubules, resulting in interstitial inflammation and migration of mononuclear cells in DSS rats, because block- ade of albuminuria by NAC counteracted the p47phox and MCP-1 expression. -

TOBAM Maximum Diversification All World Developed Ex North America USD
TOBAM Maximum Diversification All World Developed ex North America USD 31/12/2019 Instrument Weight BP PLC 0.10% IDEMITSU KOSAN CO LTD 0.21% INPEX HOLDINGS INC 0.07% JX HOLDINGS INC 0.09% NESTE OIL OYJ 1.16% OMV AG 0.08% SANTOS LTD 0.02% SBM OFFSHORE NV 0.05% TGS NOPEC GEOPHYSICAL CO ASA 0.02% VOPAK 0.02% WOOD GROUP (JOHN) PLC 0.02% AIR LIQUIDE 0.23% AIR WATER INC 0.02% AKZO NOBEL 0.12% ALUMINA LTD 0.03% AMCOR PLC-CDI 0.08% AVON RESOURCES LTD 0.53% BORAL LTD 0.02% CHR HANSEN HOLDING A/S 0.08% DAICEL CHEMICAL INDUSTRIES 0.02% DOWA HOLDINGS CO LTD 0.01% EMS-CHEMIE HOLDING AG-REG 0.03% FLETCHER BUILDING LTD 0.02% FORTESCUE METALS GROUP LTD 0.60% GIVAUDAN-REG 0.16% HITACHI CHEMICAL CO LTD 0.03% HUHTAMAKI OYJ 0.03% ISRAEL CHEMICALS LTD 0.02% JAMES HARDIE INDUSTRIES-CDI 0.07% JFE HOLDINGS INC 0.02% KANSAI PAINT CO LTD 0.03% KURARAY CO LTD 0.03% MITSUBISHI MATERIALS CORP 0.02% NEWCREST MINING LTD 1.35% TOBAM Maximum Diversification All World Developed ex North America USD 31/12/2019 Instrument Weight NIPPON PAINT CO LTD 0.05% NIPPON PAPER INDUSTRIES CO L 0.04% NIPPON SHOKUBAI CO LTD 0.01% NISSAN CHEMICAL INDUSTRIES 0.04% NOF CORP 0.02% NORTHERN STAR RESOURCES LTD 0.66% NOVOZYMES A/S-B SHARES 0.07% OJI PAPER CO LTD 0.03% ORICA LTD 0.02% ORORA LTD 0.02% SARACEN MINERAL HOLDINGS LTD 0.32% SMURFIT KAPPA GROUP PLC 0.04% SYMRISE AG 0.04% TAIHEIYO CEMENT CORP 0.02% TAIYO NIPPON SANSO CORP 0.02% TEIJIN LTD 0.02% THYSSENKRUPP AG 0.04% TORAY INDUSTRIES INC 0.02% WIENERBERGER AG 0.02% ADP 0.04% AENA SA 0.09% ALFA LAVAL AB 0.04% ALL NIPPON AIRWAYS CO LTD -
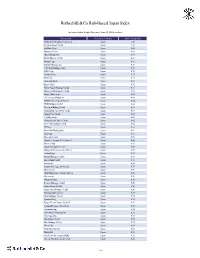
R&Co Risk-Based Japan Index
Rothschild & Co Risk-Based Japan Index Indicative Index Weight Data as of June 30, 2021 on close Constituent Exchange Country Index Weight(%) McDonald's Holdings Co Japan L Japan 1.29 Idemitsu Kosan Co Ltd Japan 1.12 SoftBank Corp Japan 1.05 Nintendo Co Ltd Japan 0.86 Hitachi Metals Ltd Japan 0.83 Yakult Honsha Co Ltd Japan 0.82 Iwatani Corp Japan 0.81 ENEOS Holdings Inc Japan 0.79 FUJIFILM Holdings Corp Japan 0.78 KDDI Corp Japan 0.75 Toshiba Corp Japan 0.73 Calbee Inc Japan 0.73 Ajinomoto Co Inc Japan 0.72 Eisai Co Ltd Japan 0.72 Nissin Foods Holdings Co Ltd Japan 0.71 Morinaga Milk Industry Co Ltd Japan 0.70 Japan Tobacco Inc Japan 0.66 H.U. Group Holdings Inc Japan 0.66 JCR Pharmaceuticals Co Ltd Japan 0.64 MEIJI Holdings Co Ltd Japan 0.64 Yamazaki Baking Co Ltd Japan 0.63 Chugoku Electric Power Co Inc/ Japan 0.63 Nippon Gas Co Ltd Japan 0.63 PeptiDream Inc Japan 0.62 Chubu Electric Power Co Inc Japan 0.62 Seven & i Holdings Co Ltd Japan 0.62 FP Corp Japan 0.61 Pola Orbis Holdings Inc Japan 0.61 Lion Corp Japan 0.61 Shiseido Co Ltd Japan 0.60 Nippon Telegraph & Telephone C Japan 0.60 Nichirei Corp Japan 0.59 Japan Post Bank Co Ltd Japan 0.59 Kobayashi Pharmaceutical Co Lt Japan 0.59 Anritsu Corp Japan 0.58 Skylark Holdings Co Ltd Japan 0.58 Kyowa Kirin Co Ltd Japan 0.58 Lawson Inc Japan 0.58 Suntory Beverage & Food Ltd Japan 0.57 Kinden Corp Japan 0.57 MS&AD Insurance Group Holdings Japan 0.56 Shimano Inc Japan 0.56 Mitsubishi Corp Japan 0.56 Zensho Holdings Co Ltd Japan 0.56 Tokai Carbon Co Ltd Japan 0.56 Japan Post Holdings Co Ltd -
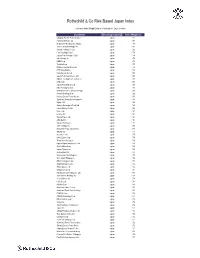
Rothschild & Co Risk-Based Japan Index
Rothschild & Co Risk-Based Japan Index Indicative Index Weight Data as of January 31, 2020 on close Constituent Exchange Country Index Weight (%) Chugoku Electric Power Co Inc/ Japan 1.01 Yamada Denki Co Ltd Japan 0.91 McDonald's Holdings Co Japan L Japan 0.88 Sushiro Global Holdings Ltd Japan 0.82 Skylark Holdings Co Ltd Japan 0.82 Fast Retailing Co Ltd Japan 0.78 Japan Post Holdings Co Ltd Japan 0.78 Ain Holdings Inc Japan 0.78 KDDI Corp Japan 0.77 Toshiba Corp Japan 0.75 Mizuho Financial Group Inc Japan 0.74 NTT DOCOMO Inc Japan 0.73 Kobe Bussan Co Ltd Japan 0.72 Japan Post Insurance Co Ltd Japan 0.69 Nippon Telegraph & Telephone C Japan 0.69 LINE Corp Japan 0.69 Japan Post Bank Co Ltd Japan 0.68 Nitori Holdings Co Ltd Japan 0.67 MS&AD Insurance Group Holdings Japan 0.66 Konami Holdings Corp Japan 0.66 Kyushu Electric Power Co Inc Japan 0.65 Sumitomo Realty & Development Japan 0.65 Fujitsu Ltd Japan 0.63 Suntory Beverage & Food Ltd Japan 0.63 Japan Airlines Co Ltd Japan 0.62 NEC Corp Japan 0.61 Lawson Inc Japan 0.60 Sekisui House Ltd Japan 0.60 ABC-Mart Inc Japan 0.60 Kyushu Railway Co Japan 0.60 ANA Holdings Inc Japan 0.59 Mitsubishi Heavy Industries Lt Japan 0.58 ORIX Corp Japan 0.57 Secom Co Ltd Japan 0.57 Seiko Epson Corp Japan 0.56 Trend Micro Inc/Japan Japan 0.56 Nippon Paper Industries Co Ltd Japan 0.56 Suzuki Motor Corp Japan 0.56 Japan Tobacco Inc Japan 0.55 Aozora Bank Ltd Japan 0.55 Sony Financial Holdings Inc Japan 0.55 West Japan Railway Co Japan 0.54 MEIJI Holdings Co Ltd Japan 0.54 Sugi Holdings Co Ltd Japan 0.54 Tokyo -

Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone?
UC Berkeley Working Paper Series Title Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone? Permalink https://escholarship.org/uc/item/00m7d34g Authors Lincoln, James R. Shimotani, Masahiro Publication Date 2009-09-24 eScholarship.org Powered by the California Digital Library University of California WHITHER THE KEIRETSU, JAPAN’S BUSINESS NETWORKS? How were they structured? What did they do? Why are they gone? James R. Lincoln Walter A. Haas School of Business University of California, Berkeley Berkeley, CA 94720 USA ([email protected]) Masahiro Shimotani Faculty of Economics Fukui Prefectural University Fukui City, Japan ([email protected]) 1 INTRODUCTION The title of this volume and the papers that fill it concern business “groups,” a term suggesting an identifiable collection of actors (here, firms) within a clear-cut boundary. The Japanese keiretsu have been described in similar terms, yet compared to business groups in other countries the postwar keiretsu warrant the “group” label least. The prewar progenitor of the keiretsu, the zaibatsu, however, could fairly be described as groups, and, in their relatively sharp boundaries, hierarchical structure, family control, and close ties to the state were structurally similar to business groups elsewhere in the world. With the break-up by the U. S. Occupation of the largest member firms, the purging of their executives, and the outlawing of the holding company structure that held them together, the zaibatsu were transformed into quite different business entities, what we and other literature call “network forms” of organization (Podolny and Page, 1998; Miyajima, 1994). -

A List of Companies and Organizations for Human Rights Due Diligence Workshop in Japan
A list of Companies and Organizations for Human Rights Due Diligence Workshop in Japan *This list shows companies and organizations that participated in Human Rights Due Diligence Workshop in Japan *Names of the participants are listed in final reports of each year`s workshop. Developed on 3rd of April, 2020 A list of Companies for the Workshop Asahi Glass Co., Ltd., ASICS Corporation, Ajinomoto Co., Inc., ANA HOLDINGS INC., ABeam Consulting Ltd., ALPSELECTRICCO.,LTD., ANRITSU CORPORATION, E-Square Inc, EQ Management Limited, AEON CO., LTD., Insight Consulting Inc., The Walt Disney Company (Japan) Ltd., SGS Japan Inc., SG Holdings Co., Ltd., NTT DATA Corporation, NTT DOCOMO, INC., KAO Corporation, Kawasaki Kisen Kaisha, Ltd., Kawasaki Heavy Industries, Ltd., Casley Consulting, Inc., Kewpie Corporation, Kyodo Printing Co., Ltd., QUICK Corp ESG Research Center, Cuore C3 Co., Ltd., KUREHA CORPORATION, KOSÉ Corporation, INPEX CORPORATION, KONICA MINOLTA, INC., SUSCOM, Sanofi K.K., YUIDEA Inc., JSR Corporation, JCB Co., Ltd., Shiseido Company, Limited, NIPPON STEEL ENGINEERING CO., LTD., Sumitomo Chemical Company, Limited, SEKISUI CHEMICAL CO., LTD., Seven & i Holdings Co., Ltd., Sony Corporation, Sompo Risk Management Inc., DAIICHI SANKYO COMPANY, LIMITED, Takenaka Corporation, Duskin Co., Ltd., CHUGAI PHARMACEUTICAL CO., LTD., TDK Corporation, Teijin Limited, Taylor Made Golf Company, Inc., Deloitte Tohmatsu Consulting LLC, TOKIO MARINE & NICHIDO RISK CONSULTING CO.,LTD., Tokyo Foundation, TOSHIBA CORPORATION, TOTO LTD., TOYO SEIKAN GROUP -

List of Donor Companies: Business Sector Emergency Donation for Earthquake Victims in Central Java, Indonesia, on May 27, 2006 (In Alphabetical Order of Companies)
List of Donor Companies: Business Sector Emergency Donation for Earthquake Victims in Central Java, Indonesia, on May 27, 2006 (in alphabetical order of companies) As of August 25, 2006 Nippon Keidanren Total amount contributed: \1,621,520,000.- Nippon Keidanren has been instrumental in soliciting business sector funds when disasters hit hard various parts of the world in the past. On May 27, 2006, central part of Java Island, Indonesia, was struck by a heavy earthquake, claiming more than 5,000 human lives and injuring almost 40,000, creating over 400,000 refugees and causing damages to more than 500,000 houses. Believing that the damages there were extensive, Nippon Keidanren initiated fund raising activities and provided solicited funds and goods such as tents and water purifiers for Indonesian people through Red Cross and NPOs under the auspice of the Japan Platform. Following is the list of donor companies that provided funds and goods through Nippon Keidanren and / or independently. 1 ABB K.K. 40 BOSCH CORPORATION 79 DAIWA HOUSE INDUSTRY CO.,LTD. 2 ACOM CO.,LTD. 41 BRIDGESTONE CORPORATION 80 DAIWA SECURITIES GROUP INC. 3 ADEKA CORPORATION 42 BROTHER INDUSTRIES,LTD. 81 DAIWABO COMPANY LIMITED 4 ADVANEX INC. 43 BUNKYODO CO.,LTD. 82 DENKI KAGAKU KOGYO K.K. 5 ADVANTEST CORPORATION 44 BUSINESS CONSULTANTS,INC. 83 DENSO CORPORATION 6 AEON 45 CALBEE FOODS CO.,LTD. 84 DENTSU INC. 7 AICHI STEEL CORPORATION 46 Canon Group 85 DHC CORPORATION 8 AICHI TOKEI DENKI CO.,LTD. 47 CAPCOM CO.,LTD. 86 DOWA MINING COMPANY,LTD. 9 AIFUL CORPORATION 48 CASIO COMPUTER CO.,LTD. -

Stoxx® Asia/Pacific 600 Ax Size Index
STOXX® ASIA/PACIFIC 600 AX SIZE INDEX Components1 Company Supersector Country Weight (%) M3 Health Care Japan 3.07 WUXI BIO Health Care Hong Kong 2.40 AFTERPAY Financial Services Australia 2.30 NIHON M&A CENTER Financial Services Japan 2.21 NORTHERN STAR Basic Resources Australia 2.05 SHENZHOU INTL.GP.HDG. Personal & Household Goods Hong Kong 1.97 Shimadzu Corp. Industrial Goods & Services Japan 1.83 TSURUHA HOLDINGS Retail Japan 1.82 Mirvac Group Real Estate Australia 1.72 TIS INC. Technology Japan 1.71 CAPCOM Technology Japan 1.64 JSR Corp. Chemicals Japan 1.62 NH FOODS Food & Beverage Japan 1.49 Santos Ltd. Oil & Gas Australia 1.48 Cochlear Ltd. Health Care Australia 1.46 Nabtesco Corp. Industrial Goods & Services Japan 1.45 TREASURY WINE ESTATES Food & Beverage Australia 1.42 Marui Group Co. Ltd. Retail Japan 1.33 Henderson Land Development Co. Real Estate Hong Kong 1.29 NICHIREI Food & Beverage Japan 1.21 Casio Computer Co. Ltd. Personal & Household Goods Japan 1.20 NET ONE SYSTEMS Technology Japan 1.19 EVOLUTION MINING Basic Resources Australia 1.18 Comsys Holdings Corp. Construction & Materials Japan 1.17 Amada Co. Ltd. Industrial Goods & Services Japan 1.16 Taiheiyo Cement Corp. Construction & Materials Japan 1.11 ITO EN Food & Beverage Japan 1.06 KAKAKU.COM Retail Japan 1.02 THK Co. Ltd. Industrial Goods & Services Japan 0.99 SUGI HOLDINGS Retail Japan 0.99 FUJI ELECTRIC Industrial Goods & Services Japan 0.99 Boral Ltd. Construction & Materials Australia 0.98 Ibiden Co. Ltd. Industrial Goods & Services Japan 0.97 Nippon Yusen K.K. -

Survey of Integrated Reports in Japan 2018
Survey of Integrated Reports in Japan 2018 Integrated Reporting Center of Excellence KPMG in Japan March 2019 home.kpmg/jp Message from global thought leaders In this, the fifth year this survey report has been issued, KPMG has solicited the observations of thought leaders on corporate reporting. Japanese businesses pride themselves on a longer-term focus compared to the rest of the world, where prioritizing short-term gains has too often become the norm. Focusing on long-term value creation and taking into account all of the resources an organization uses is the sustainable, profitable, and proven way to manage a business. Many Japanese businesses are just beginning to implement integrated thinking and reporting. The findings in this report show that senior management must take ownership to spread integrated thinking in their businesses – not just in accounting but in strategy, operations, marketing, and the rest of the company as well. This report makes me optimistic and excited for Japanese business leaders as they strive to think, act, and communicate in an integrated and sustainable way. Dominic Barton ― International Integrated Reporting Council, Chairman Integrated Reporting improves communication between companies and investors and where most effective, sets the stage for enhanced corporate value creation over the mid to long-term. Investors desire integrated reports which provide comprehensive information disclosure useful for making investment decisions. The International Corporate Governance Network (ICGN) has encouraged integrated reporting for many years. In 2015, ICGN s Disclosure and Transparency Committee ’ released Guidance on Integrated Business Reporting. The Guidance amplifies that strategic decisions should be based on factors that are broader than those reflected in the financial statements.