Predicting Habitat Availability for the Red-Billed Chough In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Impact of Human Activity on Foraging Flocks and Populations of the Alpine Chough Pyrrhocorax Graculus
Avocetta N°19: 189-193 (1995) Impact of human activity on foraging flocks and populations of the alpine chough Pyrrhocorax graculus ANNE DELESTRADE Centre de Biologie des Ecosystèmes d'Altitude, Université de Pau, 64000 Pau, France. Present address: Institut d'écologie, CNRS URA 258, Université Pierre et Marie Curie, 7 qua i St Bernard, 75252 Paris, France. Abstract - The Alpine Chough Pyrrhocorax graculus is a social corvid which uses food provided by tourist activities in mountain regions (e.g. at ski stations, refuse dumps, picnic areas). In order to determine the impact ofthe human food supply on the Alpine Chough, foraging flock size and distribution were studied in a tourist region in the Northern French Alps between 1988 and 1992. Alpine Chough attendance at tourist sites was closely related to human activities. Activity rhytbrn was influenced by human presence on picnic area in summer. Relations to human activities held at a seasonal scale (such as opening of a ski station) but not at a daily time scale (such as weekend). Long term trends of Alpine Chough populations since intense tourist development at altitude are discussed with regard of flock size counts recorded at a same site before and after intense tourist development. Introduction a little studied species, and it is particularly uncertain whether Alpine Chough populations have increased Availability of food is a factor which influences the since the intense development of tourist activities in distribution and abundance of species at a range of mountains. spatial and temporal scales. Many bird species forage The aims of this study were (1) to determine whether in human related habitats (Murton and Wright 1968), the Alpine Chough adapted its foraging behaviour to and food supplied by man (e.g. -

Warm Temperatures During Cold Season Can Negatively Affect Adult Survival in an Alpine Bird
Received: 28 February 2019 | Revised: 5 September 2019 | Accepted: 9 September 2019 DOI: 10.1002/ece3.5715 ORIGINAL RESEARCH Warm temperatures during cold season can negatively affect adult survival in an alpine bird Jules Chiffard1 | Anne Delestrade2,3 | Nigel Gilles Yoccoz2,4 | Anne Loison3 | Aurélien Besnard1 1Ecole Pratique des Hautes Etudes (EPHE), Centre d'Ecologie Fonctionnelle Abstract et Evolutive (CEFE), UMR 5175, Centre Climate seasonality is a predominant constraint on the lifecycles of species in alpine National de la Recherche Scientifique (CNRS), PSL Research University, and polar biomes. Assessing the response of these species to climate change thus Montpellier, France requires taking into account seasonal constraints on populations. However, interac- 2 Centre de Recherches sur les Ecosystèmes tions between seasonality, weather fluctuations, and population parameters remain d'Altitude (CREA), Observatoire du Mont Blanc, Chamonix, France poorly explored as they require long‐term studies with high sampling frequency. This 3Laboratoire d'Ecologie Alpine study investigated the influence of environmental covariates on the demography of a (LECA), CNRS, Université Grenoble Alpes, Université Savoie Mont Blanc, corvid species, the alpine chough Pyrrhocorax graculus, in the highly seasonal environ- Grenoble, France ment of the Mont Blanc region. In two steps, we estimated: (1) the seasonal survival 4 Department of Arctic and Marine of categories of individuals based on their age, sex, etc., (2) the effect of environ- Biology, UiT The Arctic University of Norway, Tromsø, Norway mental covariates on seasonal survival. We hypothesized that the cold season—and more specifically, the end of the cold season (spring)—would be a critical period for Correspondence Jules Chiffard, CEFE/CNRS, 1919 route de individuals, and we expected that weather and individual covariates would influence Mende, 34090 Montpellier, France. -

The Chough in Britain and Ireland
The Chough in Britain and Ireland /. D. Bullock, D. R. Drewett and S. P. Mickleburgh he Chough Pyrrhocoraxpyrrhocorax has a global range that extends from Tthe Atlantic seaboard of Europe to the Himalayas. Vaurie (1959) mentioned seven subspecies and gave the range of P. p. pyrrhocorax as Britain and Ireland only. He considered the Brittany population to be the race found in the Alps, Italy and Iberia, P. p. erythroramphus, whereas Witherby et al. (1940) regarded it as the nominate race. Despite its status as a Schedule I species, and general agreement that it was formerly much more widespread, the Chough has never been adequately surveyed. Apart from isolated regional surveys (e.g. Harrop 1970, Donovan 1972), there has been only one comprehensive census, undertaken by enthusiastic volunteers in 1963 (Rolfe 1966). Although often quoted, the accuracy of the 1963 survey has remained in question, and whether the population was increasing, stable or in decline has remained a mystery. In 1982, the RSPB organised an international survey in conjunction with the IWC and the BTO, to determine the current breeding numbers and distribution in Britain and Ireland and to collect data on habitat types within the main breeding areas. A survey of the Brittany population was organised simultaneously by members of La Societe pour 1'Etude et la Protection de la Nature en Bretagne (SEPNB). The complete survey results are presented here, together with an analysis of the Chough's breeding biology based on collected data and BTO records, along with a discussion of the ecological factors affecting Choughs. Regional totals and local patterns of breeding and feeding biology are discussed in more detail in a series of regional papers for Ireland, the Isle of Man, Wales and Scotland (Bullock*/al. -

Evolution of Corvids and Their Presence in the Neogene and the Quaternary in the Carpathian Basin
Ornis Hungarica 2020. 28(1): 121–168. DOI: 10.2478/orhu-2020-0009 Evolution of Corvids and their Presence in the Neogene and the Quaternary in the Carpathian Basin Jenő (Eugen) KESSLER Received: September 09, 2019 – Revised: February 12, 2020 – Accepted: February 18, 2020 Kessler, J. (E.) 2020. Evolution of Corvids and their Presence in the Neogene and the Quater- nary in the Carpathian Basin. – Ornis Hungarica 28(1): 121–168. DOI: 10.2478/orhu-2020-0009 Abstract: Corvids are the largest songbirds in Europe. They are known in the avian fauna of Europe from the Miocene, the beginning of the Neogene, and are currently represented by 11 species. Due to their size, they occur more frequently among fossilized material than other types of songbirds, and thus have been examined to the largest extent. In the current article, we present their known evolution and their fossilized taxa in Europe and examine the osteology of extant species. Keywords: Corvidae, Neogene, Quaternary, Europe, Carpathian Basin, osteology Összefoglalás: A varjúfélék a legnagyobb termetű, Európában is elterjedt énekesmadarak. A kontinens madárfau- nájában a neogén elejétől, a miocénból ismertek, és jelenleg 11 fajjal vannak képviselve. Termetük következtében gyakrabban előfordulnak a fosszilis anyagban, mint a többi énekesmadár típus, és ennek következtében nagyobb mértékben is tanulmányozták őket. Jelen tanulmányban bemutatjuk az ismert európai evolúciójukat és fosszilis taxonjaikat, és foglalkozunk a recens fajok csonttanával is. Kulcsszavak: Corvidae, neogén, negyedidőszak, Európa, Kárpát-medence, csonttan Department of Paleontology, Eötvös Loránd University, 1117 Budapest, Pázmány Péter sétány 1/c, Hungary, e-mail: [email protected] Introduction About half of the current avian species – if not more – consists of songbirds, which are dis- tributed all around the world apart from Antarctica with a large number of specimens. -

Federal Register/Vol. 85, No. 74/Thursday, April 16, 2020/Notices
21262 Federal Register / Vol. 85, No. 74 / Thursday, April 16, 2020 / Notices acquisition were not included in the 5275 Leesburg Pike, Falls Church, VA Comment (1): We received one calculation for TDC, the TDC limit would not 22041–3803; (703) 358–2376. comment from the Western Energy have exceeded amongst other items. SUPPLEMENTARY INFORMATION: Alliance, which requested that we Contact: Robert E. Mulderig, Deputy include European starling (Sturnus Assistant Secretary, Office of Public Housing What is the purpose of this notice? vulgaris) and house sparrow (Passer Investments, Office of Public and Indian Housing, Department of Housing and Urban The purpose of this notice is to domesticus) on the list of bird species Development, 451 Seventh Street SW, Room provide the public an updated list of not protected by the MBTA. 4130, Washington, DC 20410, telephone (202) ‘‘all nonnative, human-introduced bird Response: The draft list of nonnative, 402–4780. species to which the Migratory Bird human-introduced species was [FR Doc. 2020–08052 Filed 4–15–20; 8:45 am]‘ Treaty Act (16 U.S.C. 703 et seq.) does restricted to species belonging to biological families of migratory birds BILLING CODE 4210–67–P not apply,’’ as described in the MBTRA of 2004 (Division E, Title I, Sec. 143 of covered under any of the migratory bird the Consolidated Appropriations Act, treaties with Great Britain (for Canada), Mexico, Russia, or Japan. We excluded DEPARTMENT OF THE INTERIOR 2005; Pub. L. 108–447). The MBTRA states that ‘‘[a]s necessary, the Secretary species not occurring in biological Fish and Wildlife Service may update and publish the list of families included in the treaties from species exempted from protection of the the draft list. -

Journal of Alpine Research | Revue De Géographie Alpine
Journal of Alpine Research | Revue de géographie alpine 98-4 | 2010 La montagne, laboratoire du changement climatique Mountains, the climate change laboratory Édition électronique URL : http://journals.openedition.org/rga/1269 DOI : 10.4000/rga.1269 ISSN : 1760-7426 Éditeur : Association pour la diffusion de la recherche alpine, UGA Éditions/Université Grenoble Alpes Référence électronique Journal of Alpine Research | Revue de géographie alpine, 98-4 | 2010, « La montagne, laboratoire du changement climatique » [En ligne], mis en ligne le 20 décembre 2010, consulté le 02 octobre 2020. URL : http://journals.openedition.org/rga/1269 ; DOI : https://doi.org/10.4000/rga.1269 Ce document a été généré automatiquement le 2 octobre 2020. La Revue de Géographie Alpine est mise à disposition selon les termes de la licence Creative Commons Attribution - Pas d'Utilisation Commerciale - Pas de Modification 4.0 International. 1 La montagne est un territoire d’exception, réservoir de multiples ressources (bois, minerais, eau, énergie, neige, etc.), mais qui doit composer avec des handicaps naturels (pente, altitude et climat). Elle s’est ainsi construite au fil de son histoire une place spécifique au sein de l’Aménagement du territoire (RTM, Plan neige, loi « Montagne »). La question du changement climatique s’ajoute aujourd'hui aux réflexions et aux pratiques d’aménagement. Il faut alors s’interroger sur les implications de la rencontre entre un objet aux spécificités marquées et un phénomène générique, dont la représentation découle d’une appréhension globale du climat (réchauffement planétaire ou global warming) à travers des modèles prospectifs sans finesses dans leurs projections locales. L’objectif de ce numéro thématique de la RGA est d’explorer cette rencontre selon quatre domaines particuliers : écosystèmes de montagne, agropastoralisme, forêt et sylviculture, risques – naturels et économiques. -

Mount Everest, the Reconnaissance, 1921
MOUNT EVEREST The Summit. Downloaded from https://www.greatestadventurers.com MOUNT EVEREST THE RECONNAISSANCE, 1921 By Lieut.-Col. C. K. HOWARD-BURY, D.S.O. AND OTHER MEMBERS OF THE MOUNT EVEREST EXPEDITION WITH ILLUSTRATIONS AND MAPS LONGMANS, GREEN AND CO. 55 FIFTH AVENUE, NEW YORK LONDON: EDWARD ARNOLD & CO. 1922 Downloaded from https://www.greatestadventurers.com PREFACE The Mount Everest Committee of the Royal Geographical Society and the Alpine Club desire to express their thanks to Colonel Howard-Bury, Mr. Wollaston, Mr. Mallory, Major Morshead, Major Wheeler and Dr. Heron for the trouble they have taken to write so soon after their return an account of their several parts in the joint work of the Expedition. They have thereby enabled the present Expedition to start with full knowledge of the results of the reconnaissance, and the public to follow the progress of the attempt to reach the summit with full information at hand. The Committee also wish to take this opportunity of thanking the Imperial Dry Plate Company for having generously presented photographic plates to the Expedition and so contributed to the production of the excellent photographs that have been brought back. They also desire to thank the Peninsular and Oriental Steam Navigation Company for their liberality in allowing the members to travel at reduced fares; and the Government of India for allowing the stores and equipment of the Expedition to enter India free of duty. J. E. C. EATON Hon. A. R. } Secretaries. HINKS Downloaded from https://www.greatestadventurers.com CONTENTS PAGE INTRODUCTION. By SIR FRANCIS YOUNGHUSBAND, K.C.S.I., K.C.I.E., President of the Royal Geographical Society 1 THE NARRATIVE OF THE EXPEDITION By LIEUT.-COL. -

HOST LIST of AVIAN BROOD PARASITES - 2 - CUCULIFORMES; Cuculidae
C u c k o o h o s ts - p a g e 1 HOST LIST OF AVIAN BROOD PARASITES - 2 - CUCULIFORMES; Cuculidae Peter E. Lowther, Field Museum version 12 Sep 2011 This list remains a working copy; colored text used often as editorial reminder; strike-out gives indication of alternate names. Names prefixed with “&” or “%” usually indicate the host species has successfully reared the brood parasite. Notes following names qualify host status or indicate source for inclusion in list. Note on taxonomy. Cuckoo taxonomy follows Payne 2005. Most English and scientific names for hosts from Sibley and Monroe (1990); in large families, the subfamilies and tribes of Sibley and Monroe are treated as “families” in this listing of host species. Hosts listed at subspecific level indicate that that taxon sometimes considered specifically distinct (see notes in Sibley and Monroe 1990). Clamator Clamator Kaup 1829, Skizzirte Entwicklungs-Geschichte und natüriches System der Europäischen Thierwelt ... , p. 53. Chestnut-winged Cuckoo, Clamator coromandus (Linnaeus 1766) Systema Naturae, ed. 12, p. 171. Distribution. – Southern Asia. Host list. – Based on Friedmann 1964; see also Baker 1942: CORVIDAE Dicruridae Fork-tailed Drongo, Dicrurus adsimilis MUSCICAPIDAE Turdidae Orange-headed Thrush, Zoothera citrina Blue Whistling-Thrush, Myiophonus caeruleus A Black-breasted Thrush, Turdus dissimilis B Saxicolidae Oriental Magpie-Robin, Copsychus saularis Slaty-backed Forktail, Enicurus schistaceus SYLVIIDAE Garrulacidae White-crested Laughingthrush, Garrulax leucolophus Greater -
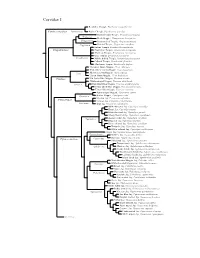
Corvidae Species Tree
Corvidae I Red-billed Chough, Pyrrhocorax pyrrhocorax Pyrrhocoracinae =Pyrrhocorax Alpine Chough, Pyrrhocorax graculus Ratchet-tailed Treepie, Temnurus temnurus Temnurus Black Magpie, Platysmurus leucopterus Platysmurus Racket-tailed Treepie, Crypsirina temia Crypsirina Hooded Treepie, Crypsirina cucullata Rufous Treepie, Dendrocitta vagabunda Crypsirininae ?Sumatran Treepie, Dendrocitta occipitalis ?Bornean Treepie, Dendrocitta cinerascens Gray Treepie, Dendrocitta formosae Dendrocitta ?White-bellied Treepie, Dendrocitta leucogastra Collared Treepie, Dendrocitta frontalis ?Andaman Treepie, Dendrocitta bayleii ?Common Green-Magpie, Cissa chinensis ?Indochinese Green-Magpie, Cissa hypoleuca Cissa ?Bornean Green-Magpie, Cissa jefferyi ?Javan Green-Magpie, Cissa thalassina Cissinae ?Sri Lanka Blue-Magpie, Urocissa ornata ?White-winged Magpie, Urocissa whiteheadi Urocissa Red-billed Blue-Magpie, Urocissa erythroryncha Yellow-billed Blue-Magpie, Urocissa flavirostris Taiwan Blue-Magpie, Urocissa caerulea Azure-winged Magpie, Cyanopica cyanus Cyanopica Iberian Magpie, Cyanopica cooki Siberian Jay, Perisoreus infaustus Perisoreinae Sichuan Jay, Perisoreus internigrans Perisoreus Gray Jay, Perisoreus canadensis White-throated Jay, Cyanolyca mirabilis Dwarf Jay, Cyanolyca nanus Black-throated Jay, Cyanolyca pumilo Silvery-throated Jay, Cyanolyca argentigula Cyanolyca Azure-hooded Jay, Cyanolyca cucullata Beautiful Jay, Cyanolyca pulchra Black-collared Jay, Cyanolyca armillata Turquoise Jay, Cyanolyca turcosa White-collared Jay, Cyanolyca viridicyanus -

Conservation Strategy for Red-Billed Choughs in Scotland: Assessment of the Impact of Supplementary Feeding and Evaluation of Future Management Strategies
Scottish Natural Heritage Research Report No. 1152 Conservation strategy for red-billed choughs in Scotland: Assessment of the impact of supplementary feeding and evaluation of future management strategies RESEARCH REPORT Research Report No. 1152 Conservation strategy for red-billed choughs in Scotland: Assessment of the impact of supplementary feeding and evaluation of future management strategies For further information on this report please contact: Jessica Shaw Scottish Natural Heritage Stilligarry SOUTH UIST HS8 5RS Telephone: 0131 314 4187 E-mail: [email protected] This report should be quoted as: Trask, A.E., Bignal, E., Fenn, S.R., McCracken, D.I., Monaghan, P. & Reid, J.M. 2020. Conservation strategy for red-billed choughs in Scotland: Assessment of the impact of supplementary feeding and evaluation of future management strategies. Scottish Natural Heritage Research Report No. 1152. This report, or any part of it, should not be reproduced without the permission of Scottish Natural Heritage. This permission will not be withheld unreasonably. The views expressed by the author(s) of this report should not be taken as the views and policies of Scottish Natural Heritage. © Scottish Natural Heritage 2020. RESEARCH REPORT Summary Conservation strategy for red-billed choughs in Scotland: Assessment of the impact of supplementary feeding and evaluation of future management strategies Research Report No. 1152 Project No: 115669, 117001 Contractor: Professor Jane Reid, University of Aberdeen Year of publication: 2020 Keywords supplementary feeding; parasites; habitat management; inbreeding; conservation translocations; Population Viability Analysis; red-billed choughs Background The red-billed chough (Pyrrhocorax pyrrhocorax) is a Species of European Conservation Concern (Annex 1, EC Birds Directive) and a Schedule 1 species in Scotland and the UK (Wildlife & Countryside Act 1981), with the UK Pyrrhocorax pyrrhocorax pyrrhocorax subspecies listed as Amber. -

The White-Throated Magpie-Jay
THE WHITE-THROATED MAGPIE-JAY BY ALEXANDER F. SKUTCH ORE than 15 years have passed since I was last in the haunts of the M White-throated Magpie-Jay (Calocitta formosa) . During the years when I travelled more widely through Central America I saw much of this bird, and learned enough of its habits to convince me that it would well repay a thorough study. Since it now appears unlikely that I shall make this study myself, I wish to put on record such information as I gleaned, in the hope that these fragmentary notes will stimulate some other bird- watcher to give this jay the attention it deserves. A big, long-tailed bird about 20 inches in length, with blue and white plumage and a high, loosely waving crest of recurved black feathers, the White-throated Magpie-Jay is a handsome species unlikely to be confused with any other member of the family. Its upper parts, including the wings and most of the tail, are blue or blue-gray with a tinge of lavender. The sides of the head and all the under plumage are white, and the outer feathers of the strongly graduated tail are white on the terminal half. A narrow black collar crosses the breast and extends half-way up each side of the neck, between the white and the blue. The stout bill and the legs and feet are black. The sexes are alike in appearance. The species extends from the Mexican states of Colima and Puebla to northwestern Costa Rica. A bird of the drier regions, it is found chiefly along the Pacific coast from Mexico southward as far as the Gulf of Nicoya in Costa Rica. -

Longevity of Birds in Captivity
Vol.1928 XLV] I BROW•,Longevity ofBirds in Captivity. 345 LONGEVITY OF BIRDS IN CAPTIVITY. BY C. EMERSON BROWN. BUT little is known of the length of life of birds. Bird banders are doing some valuable work along these lines, but even their data must necessarilybe very incomplete. It is difficultto state how muchlonger a bird will live in the wild than in captivity. In the wild state very little is knownof the durationof life. It is, however,extremely doubtful if any birds live long enoughto die of old age, as their lives are traveledon a road besetwith dangers, and eternal vigilanceand hard work are the price of living. Thousandsof birds meet violent deaths yearly either by being killedby otherbirds, mammals or reptiles,flying against buildings or wiresduring their migration,or are shot by hunters. Besides this, many perishbecause of the vicissitudesof the weatherand lack of food. They certainly have many advantagesin captivity, the greatestof whichis the protectionfrom their enemies. There is never a scarcityof food or drink and they are protectedfrom severeweather at all times. It is alsoa fact that somespecimens in captivitydo die of diseasesof theheart, blood and kidneys which are diseasesso commonin human old age. It is, however, difficult to say whether the length of life is shortenedby captivityor not. I have,prepared the followinglist showingthe duration of life of a few speciesin captivity in the PhiladdphiaZoological Garden, which I hopewill be of interest. Among the Passefinebirds the Yellow-headedBlackbird heads the list with 18 years,the JungleBabbler, 16 and the Himalayan Chough,14. The Hyacinth Macaw lived 27 years and a Horn- bill 24.