Chexical Society
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

606 Lan Private Residents. (Surrey
606 LAN PRIVATE RESIDENTS. (SURREY. Lane Maj.-Gen. Charles 8., C.B. Harcourt•Langridge Alfred, Selbourne, New Haw, Lasenby Ahvyn, The Old house, Porta ho. Primley rd. York Town, Camberley AddlestonE> mouth road, Esher Lane Col. Cl&yton Turner C.I.E. Down- Langridge A. 8. 8 Galpin's rd. Norbry SW Lash Jn . .A..The Bank, Manor rd.Wallngtn fold, Albury road, Guildford Langridge G. T. Foxlea, Church st. Epsom La~ham Montague Richard, 13 Tabor gro. Lane A. 208 Worple rd. Wimbledon SW Langridge Renben John, 6 Strathyre aven. Wimbledon SW iLane A. 28 Dagnall pk. Sth. Norwood SE Norbury SW Lassam Hy. Olifiord, The Hut, Burwood Lane 0. A. Hedley, York road, Guildford Langridge Wm. 403 Brighton rd. Croydon Park road, Hersham, Walton-on-Thames Lane Chas. J. R. 47 Pagoda av. Richmond· Langridge William T. The Meadows, Last A. E. 36 Brigstock rd. Thornton Heath Lane Edwd. Jn. 95 Castelnan, Barnes SW Maiden road, Cheam, Sutton Last Arthur William, Bolton house, Wor. Lane G. Hill brow, Dalmeny rd. Wallngtn Langrish Capt. Thomas (late R.A.), Sylvan cester road, Chearo, Sutton Lane Geo. The Laurels, Charlwood, Horley lodge, Heath End, Farnham Last C. H. 82 Woodside, Wimbledon SW Lane H. Mon Abri, Milbourne la. Esher Langrish J. 0. 11 Stanford rd. NorburySW r~ast C. St. Ives, Cornwall rd.Cheam,Suttn Lane Henry, 41 The Crescent, Croydon Langrish Jn. 19 Osmond gdns. Wallington Last E.W.Annandale, Low.Bourne,Farnhm Lane Henry John, Holmwood, Carshalton Langrish Rd.Cloverlea, Bramley, Guildford Last F. W. 3 Eldon pk. Sth. -

Buses from Raynes Park Continues to N87 Aldwych for Covent Garden
Buses from Raynes Park continues to N87 Aldwych for Covent Garden 24 hour 57 service Clapham Park Atkins Road Wandsworth Town Centre Ridgway Streatham Hill Copse Hill Ridgway Wimbledon Ridgway Telford Avenue Woodhayes Road Edge Hill Telephone Exchange Wimbledon Village Wimbledon Wimbledon Hill Road Park Copse Hill Copse Hill for Wimbledon Atkinson Close Cottenham Park Road West Wimbledon Route finder Christ Church Worple Road Wimbledon Streatham Hill Francis Grove Bus Station Day buses including 24-hour routes Worple Road Spencer Hill 163 Bus route Towards Bus stops Cottenham Park Road Worple Road Wimbledon Pepys Road Darlaston Road Streatham 24 hour Clapham Park St Leonard’s Church 57 service Worple Road WIMBLEDON Alexandra Road Lower Downs Road Kingston Durham Road St George’s Road Orchard Lane Kingston +DUWÀHOG5RDG 131 Sir Cyril Black Way STREATHAM D ROAD OA S R ARTER Worple Road D VE Mitcham Lane OA DAR Albert Grove Tooting Broadway E R PEN D IDG TANA A BR N O BERR CAM R Wimbledon AD MO E +DUWÀHOG5RDG RO PEP R N O Y Police Station WY M R Bertram Cottages L E D OA New Malden KEN Y A N S U A MERTON N 152 ROA D D G O The Broadway D R Nelson Haydons Road A H Kingston Road O A D R E Wimbledon Theatre D M L Hospital Wilton Crescent U P Pollards Hill T R F R ROSEVINE RE O R A O Southcroft Road K H A TO W D L M W AD A L IN O I VE R Wimbledon Chase K5 T ER M L C ON NC Y A Merton Park SPE RN E T Morden N RO The Broadway G M A Ham R 163 ROAD R B E ST O O T R AD Kingston Road Polka Theatre Dukes Avenue V O OA Mostyn Road -

Raynes Park January 2012
Part II Potential sites for new uses Raynes Park January 2012 Introduction Have your say Please tell us what you think about the potential sites for This document is a reduced form of the Sites and new uses by Friday 23 March 2012 by responding in Polices Part II – potential sites for new uses to show writing by post or e‐mail to: only those sites which lie within or close to Raynes Park. Strategic Policy and Research Future Merton Please note that page numbering and proposal London Borough of Merton numbers remains consistent with the unabridged th original document. 12 Floor Civic Centre London Road, Morden. SM4 5DX. Email: [email protected] Telephone: 020 8545 4141/ 020 8545 3837 If you are part of a community group, business forum or other organisation and would like someone to attend to explain the site assessments, please contact us by telephone at: 020 8545 4141/020 8545 3837, by e‐mail at [email protected] or by post at: Strategic Policy and Research, Future Merton, London Borough of Merton, 12th Floor Civic Centre, London Road, Morden, SM4 5DX and we will do our best to meet your request. Part II Content What’s happened so far?..........................................3 Have your say............................................................3 What will happens next? ..........................................3 Notes on site assessments........................................4 Ensuring quality in Merton .......................................4 Sites 01 – “P3” Hartfield Road Car Park.................................... 6 33 – Elm Nursery Car Park ..............................................60 02 – 43‐45 Palestine Grove .............................................. 8 34 – Raleigh Gardens Car Park........................................62 04 – Bond Road Day Nursery.......................................... 10 35 – Mitcham Fire Station ..............................................64 05 – Colliers Wood Community Centre......................... -

Buses from Wimbledon Village
Buses from Wimbledon Village River Thames Route finder 493 Richmond North Sheen Day buses including 24-hour services Richmond Manor Circus Bus Station RICHMOND Bus route Towards Bus stops Sheen Road 24 hour 93 service Queens Road for North Sheen 24 hour North Cheam Putney Bridge 93 service East Sheen Putney Bridge Sheen Lane for Mortlake PUTNEY ROEHAMPTON 200 Mitcham Roehampton Lane Roehampton Putney Roehampton Lane University of Surrey Earl Spencer Putney High Street Raynes Park Rosslyn Park R.F.C Barnes Common Queen Mary’s Roehampton Lane University Hospital Putney Heath 493 North Sheen Green Man River Thames Tooting Tibbet’s Ride Princes Way Tibbet’s Corner West Hill Beaumont Road Southmead Beaumont Road Stapleford Close Primary School Beaumont Road Southmead Linstead Way Road Augustus Road Wimbledon Parkside Albemarle 6RXWKÀHOGV Wimbledon Parkside Wimbledon Park Road Queensmere Road Southdean Gardens Wimbledon Park Road Woodspring Road Wimbledon Tennis Club and Museum Parkside Hospital Church Road Somerset Road All England m S O Lawn M E Tennis R S CA E Club LO T N R N O E A Z D P ROAD ARK B U R G H L SID E Y R OA l E E D ENU Wimbledon V D A A D E O [ R D Common T A I A KS Y O Tooting Broadway PAR R n R TOOTING R S A T R H . M D C M B R R E p A THE U L O R V A Y Tooting \ E D The yellow tinted area includes every CAUSEWAY H DERE S CASTER C k T St George’s H bus stop up to about one-and-a-half ] A D E LAN V Hospital A G H ENUE miles from Wimbledon Village. -

Colliers Wood
Proposed Changes to the Merton Sites and Policies Environmental Maps – Colliers Wood November 2020 This document provides details of the proposed boundary changes to the following maps currently designated in the Merton Sites and Policies Plan (2014) in the Colliers Wood neighbourhood: - Metropolitan Open Land (MOL) - Open Space - Green Corridors - Sites of Importance for Nature Conservation (SINC) Please note: 1. The pages below show extracts of the proposed changes. Refer to the Merton Sites and Policies Plan (2014) webpage for a copy of the current adopted policy maps: https://www2.merton.gov.uk/environment/planning/planningpolicy/localplan/sit esandpoliciesplan.htm 2. The following pages include the following: a. A list of all the sites designated in the 2014 Sites and Policies Map and an indication of whether there is a proposed change for each site. b. The individual maps showing the proposed change. c. Supporting text from The Environment Partnership (labelled as TEP), or London Borough of Merton Future Merton team (labelled as LBM) explaining the reasons for the proposed change. Proposed Changes to the Environmental Policy Maps These Open Spaces, Sites of Importance for Nature Conservation, Sites of Special Scientific Interest and Green Corridors are illustrated on the Policies Map and have been broken down by neighbourhood in the tables below. Colliers Wood Open Space - Education Site Name Proposed Boundary Area Boundary Change Change Ref. S017 Merton Abbey School (now Merton Yes LBM-64 Colliers Wood Abbey and Harris Academy Wimbledon) S040 Garfield School, Garfield Road No N/A Colliers Wood S061 Singlegate Primary School Yes LBM-56 Colliers Wood S064 All Saints C of E Primary School, Yes LBM-50 Colliers Wood East Road Open Space - All Other Open Spaces Site Name Proposed Boundary Area Boundary Change Change Ref. -

Cottenham Park Road Area
Proposed Controlled Parking Zone Councillor Andrew Judge Cabinet Member for (CPZ) RPC1 - Cottenham Park Road Environmental Sustainability Area. and Regeneration T: 020 8545 3425 E: [email protected] ISSUE Date : 14 MAY 2015 Dear Resident / Business The Council is required to give weight to the nature and The purpose of this leaflet is to offer residents of content of your representations and not necessarily the Cottenham Drive, Cottenham Park Road, Cottenham quantity. Your reasons are, therefore, important to us. Place, Cranford Close, Hampton Close, Heights Close, Hillview and Oakwood Road a further opportunity to All representations along with Officers’ comments comment on proposals to include these roads in the and recommendations will be presented in a report to RPC CPZ that has recently been implemented. This the Cabinet Member for Environmental Sustainability is due to representations and petitions received from and Regeneration. Please note that responses to any some residents of Cottenham Park Road and Oakwood representations received will not be made until a final Road during the implementation of RPC CPZ. decision is made by the Cabinet Member. we will also welcome representations in support. The Council is, therefore, consulting residents of your road on its intention to introduce Controlled Parking A copy of the proposed Traffic Management Orders Zone (CPZ) into the following roads: Cottenham Drive, (TMOs), a plan identifying the areas affected by the Cottenham Park Road, Cottenham Place, Cranford proposals and the Council’s Statement of Reasons can be Close, Hampton Close, Heights Close, Hillview and inspected at Merton Link, Merton Civic Centre, London Oakwood Road to operate Monday to Friday between Road, Morden, Surrey, SM4 5DX during the Council’s 12 and 1pm. -

The Collaborative City
the londoncollaborative The Collaborative City Working together to shape London’s future March 2008 THE PROJECT The London Collaborative aims to increase the capacity of London’s public sector to respond to the key strategic challenges facing the capital. These include meeting the needs of a growing, increasingly diverse and transient population; extending prosperity while safe- guarding cohesion and wellbeing, and preparing for change driven by carbon reduction. For more information visit young- foundation.org/london Abbey Wood Abchurch Lane Abchurch Yard Acton Acton Green Adams Court Addington Addiscombe Addle Hill Addle Street Adelphi Wharf Albion Place Aldborough Hatch Alder- manbury Aldermanbury Square Alderman’s Walk Alders- brook Aldersgate Street Aldersgate Street Aldgate Aldgate Aldgate High Street Alexandra Palace Alexandra Park Allhal- lows and Stairs Allhallows Lane Alperton Amen Corner Amen CornerThe Amen Collaborative Court America Square City Amerley Anchor Wharf Angel Working Angel Court together Angel to Court shape Angel London’s Passage future Angel Street Arkley Arthur Street Artillery Ground Artillery Lane Artillery AperfieldLane Artillery Apothecary Passage Street Arundel Appold Stairs StreetArundel Ardleigh Street Ashen Green- tree CourtFORE WAustinORD Friars Austin Friars Passage4 Austin Friars Square 1 AveINTRO MariaDUctio LaneN Avery Hill Axe Inn Back6 Alley Back of Golden2 Square OVerVie WBalham Ball Court Bandonhill 10 Bank Bankend Wharf Bankside3 LONDON to BarbicanDAY Barking Barkingside12 Barley Mow Passage4 -
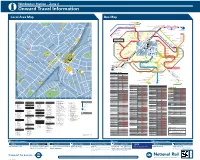
Local Area Map Bus Map
Wimbledon Station – Zone 3 i Onward Travel Information Local Area Map Bus Map 19 St. Mary’s Putney Bridge 25 93 18 40 1 C of E Church Wimbledon Park 16 7 D 17 S N 70 S E D A D D A 4 T Fellowship L A K E R O A D L R A O WALDEMAR ROAD O A . R River Thames G R 8 House E R O D O R A R O A D 1 N E M R R T O R U A St. Mary’s Church Putney Pier 219 4 S 26 E Clapham Junction Falcon Lane A A H C T P H Queenstown R T Nine Elms N 1 R KENILWORTH AVENUE A H Y A A Putney Exchange L O R 31 ’ Road C S T Lane 156 S 70 Queen Mary’s Roehampton L 1 PUTNEY R R 56 Putney CLAPHAM JUNCTION Westminster U O D University Hospital Medfield Street 60 14 A 59 Battersea Park 23 E H D U R Leopold RoadRoa 2 Clapham Junction RICARDS ROAD N O 36 G A P R O A D Lavender Hill Vauxhall 1 C E A ST. AUBYN’S AVENUE V 1 D 58 Bishop Gilpin A N87 L D O 85 Putney Heath Green Man Wandsworth Town Hall 85 22 15 P 1 Roehampton Primary School O 1 E 113 27 continues to 3 LANCASTER AVENUE L University Wandsworth LANCASTER ROAD 1 MARRYAT ROAD 106 Trafalgar Square 9 Wandsworth Southside Road A L A N R O A D Roehampton Lane Clapham Junction Northcote for Charing Cross 1 18 Tibbet’s Corner Rosslyn Park Rugby Club and Aldwych S Southfields Merton Road Southfields Academy N 1 D E O 1 D E L A D O R East Sheen A 26 10 H R D 1 38 O G 25 Durnsford Road Stroud Road LANCASTER ROAD U E D I Upper Richmond Road West Parkside Hospital S 2 K R Battersea Rise 12 E A A Wimbledon Park Road L N C S R U BELVEDERE AVENUE R S H L E M O B D Southdean Gardens E R D S HELME CLOSE Sheen Road Manor Road E E P 1 150 Wimbledon -
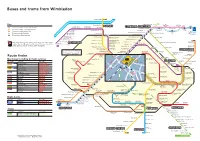
Buses and Trams from Wimbledon
Buses and trams from Wimbledon Putney Bridge 93 River Thames Clapham Junction Falcon Lane 219 Key Putney High Street Queenstown Nine Elms PUTNEY Road Lane 156 Ø— Queen MaryÕs Roehampton Putney CLAPHAM JUNCTION Westminster Connections with London Underground University Hospital Medfield Street Battersea Park Clapham Junction Lavender Hill Vauxhall u Connections with London Overground N87 Putney Heath Green Man Wandsworth Town Hall Cedars Road Roehampton continues to R Connections with National Rail University Wandsworth Wandsworth Southside Shopping Centre Trafalgar Square Clapham Junction Northcote Road for Charing Cross h Connections with Tramlink Barnes Common TibbetÕs Corner Roehampton Lane and Aldwych  Southfields Southfields Merton Road Connections with river boats East Sheen Sheen Lane for Mortlake Parkside Hospital Durnsford Road Stroud Road Wimbledon Park Road Battersea Rise Sheen Road Manor Road Southdene Gardens Wimbledon Park Arthur Road for North Sheen Parkside Calonne Road Wimbledon Park Road 57 A Woodspring Road Richmond Durnsford Road Pitt Crescent Clapham Park Red discs show the bus stop you need for your chosen bus Wimbledon Tennis Club Wandsworth Common North Side Atkins Road RICHMOND Parkside Parkside Avenue Plough Lane !A Richmond and Museum Gap Road Plough Lane Haydons Road service. The disc appears on the top of the bus stop in the Manor Circus for North Sheen 1 2 3 Church Road 4 5 6 street (see map of town centre in centre of diagram). 493 Gap Road Avondale Road Streatham Hill Wimbledon War Memorial Somerset Road Telford Avenue Wimbledon Trinity Road Church Road Alexandra Road Stadium Wimbledon Village Belvedere Avenue Marryat Road Strathearn Road STREATHAM The yellow tinted area includes every bus stop up to one-and-a-half miles Wimbledon Village Wimbledon Queens Road Wimbledon Road Streatham Hill High Street Magistrates Court Trinity Road Hazelhurst Estate Wandsworth Common from Wimbledon. -

A Wonderful Example of a Family Home in West
A WO NDERFUL EXAMPLE OF A FAMILY HOME IN WEST WIMBLEDON. PENDA RVES ROAD WEST WIMBLEDON, LONDON, SW20 8TS Unfurnished, £3,350 pcm + £285 inc VAT tenancy paperwork fee and other charges apply.* Available from 11/09/2018 A WO NDERFUL EXAMPLE OF A FAMILY HOME IN WEST WIMBLE DON. PENDA RVES ROAD WEST WIMBLEDON, LONDON, SW20 £3,350 pcm Unfurnished • 4 Bedrooms • 2 Bathrooms • Lovely double reception • Modern kitchen/breakfast room • Cloakroom • Neutral decor throughout • Modern feel • Landscaped garden • EPC Rating = D • Council Tax = F Situation Pendarves Road is one of a collection of attractive Edwardian streets within the Lambton Road conservation area. The property is within 500m of Raynes Park rail station which provides a rail link to central London within 20 minutes with a regular service both during peak and off peak hours. Within 800m are a useful Sainsbury local and Waitrose with a number of coffee shops and restaurants locally. Hollymount Primary School is found on the neighbouring street with good access to the delightful Holland Gardens and Cottenham Park. Wimbledon Village and Common are both around one mile away. Description A fantastic four bedroom family house with great reception space and lovely garden located in the heart of West Wimbledon approx 0.3 miles to the mainline station. The property benefits from a great double reception, modern kitchen/breakfast room, three good sized double bedrooms, fourth smaller double/study, two bath/shower rooms and landscaped garden. Viewing Strictly by appointment with Savills. FLOORPLANS Gross internal area: 1411 sq ft, 131.1 m² savills.co.uk Energy Performance A copy of the full Energy Performance Certificate is available on request. -

117B Cottenham Park Road
117b Cottenham Park Road West Wimbledon, London GUIDE PRICE £675,000 SUBJECT TO CONTRACT We are pleased to offer this well presented quarter house, located in a residential area within easy reach of the many excellent shops, bars and restaurants of both Wimbledon and Raynes Park. The property, offers approximately 855 ft2 of accommodation and is ideal for a young family or professional couple. WWW.COOMBERESIDENTIAL.COM 117b Cottenham Park Road West Wimbledon, London, SW20 0DS Location Cottenham Park Road is conveniently located close to Wimbledon and Kingston Town Centres with their excellent shopping facilities, as is the A3 trunk road offering fast access to Central London and both Gatwick and Heathrow airports via the M25 motorway network. Train stations at Raynes Park provide frequent services to Waterloo with its underground links to points throughout the City, with the nearest tube station being Wimbledon. The immediate area offers a wide range of recreational facilities including two parks with children’s play area and tennis courts, three golf courses, David Lloyd leisure complex and a squash club. Wimbledon Common and Richmond Park, both areas of outstanding beauty, provide a picturesque setting for picnics, go horse riding, jogging or just leisurely walks. Theatres at Richmond and Wimbledon are also popular alternatives to the West End together with an excellent choice of restaurants. There are also numerous schools for all ages, both Public and State and International. Accommodation Comprises Entrance Hall | Reception Room | Kitchen/Breakfast Room | Utility Room | Guest Cloakroom | Two Bedrooms | Family Bathroom Amenities Include Gas Fired Central Heating and Hot Water | Double Glazing | Pretty South Facing Rear Garden with Patio Area | Ideal Location The Property The front door of the property is approached from the road via a paved path and through a wrought iron gate. -

Venue Directory of Community Spaces for Hire in Merton (Information Correct As at October 2018)
Produced by Wimblecomm& Wimbledon Community Association https://wimbledoncommunity.org Venue Directory of Community Spaces for Hire in Merton (Information correct as at October 2018) Produced by Wimblecomm and the Wimbledon Community Association - https://wimbledoncommunity.org If there are any other spaces for hire within Merton that you would like us to add, do get in touch via our website. Alternatively, if your venue is listed and you would like to change the information or develop a featured listing with images and more information, you can List Your Venue directly on Wimblecomm or get in touch with us via email at [email protected]. DIRECTORY OF SPACES FOR HIRE IN MERTON (Information correct as at October 2018) VENUE NAME ADDRESS WEBSITE CONTACT PHONE/EMAIL COLLIERS WOOD Christ Church, Colliers Christ Church, 58 Christchurch Road, 07807 488 594 Wood Colliers Wood, London SW19 2NY http://www.christchurchsw19.org.uk/whos-who/ /[email protected] Colliers Wood 66-72 High Street, Colliers Wood, Community Centre SW19 2BY 020 8543 6470 / [email protected] 105 High Street Colliers Wood, https://wimbledoncommunity.org/venues/community- 0208 274 5799 / Colliers Wood Library Merton, SW19 2BW space/colliers-wood-library-2/ [email protected] The Colour House Theatre, Merton Abbey Mills, Watermill Way, SW19 https://wimbledoncommunity.org/venues/theatre/the- Color House Theatre 2RD colour-house-theatre/ 0208 542 5511 Oasis Church, Colliers Oasis Church, 1-27 Fortescue Road, 020 8287 0444 / Wood Colliers