Son Hypothesis and WD Hamilton'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Linkage Disequilibrium in Human Ribosomal Genes: Implications for Multigene Family Evolution
Copyright 0 1988 by the Genetics Societyof America Linkage Disequilibrium in Human Ribosomal Genes: Implications for Multigene Family Evolution Peter Seperack,*” Montgomery Slatkin+ and Norman Arnheim*.* *Department of Biochemistry and Program in Cellular andDevelopmental Biology, State University of New York, Stony Brook, New York I 1794, ?Department of Zoology, University of California, Berkeley, California 94720, and *Department of Biological Sciences, University of Southern California, Los Angeles, California 90089-0371 Manuscript received January8, 1988 Revised copy accepted April 2 1, 1988 ABSTRACT Members of the rDNA multigene family withina species do not evolve independently,rather, they evolve together in a concerted fashion.Between species, however, each multigenefamily does evolve independently indicating that mechanisms exist whichwill amplify and fix new mutations bothwithin populations and within species. In order to evaluate the possible mechanisms by which mutation, amplification and fixation occurwe have determined thelevel of linkage disequilibrium betweentwo polymorphic sites in human ribosomal genes in five racial groups and among individuals within two of these groups.The marked linkage disequilibriumwe observe within individuals suggeststhat sister chromatid exchangesare much more important than homologousor nonhomologous recombination events in the concerted evolution of the rDNA family and further that recent models of molecular drive may not apply to the evolution of the rDNA multigene family. HE human ribosomal gene family is composed of ferences among individualsin the geneticcomposition T approximately 400 members which arear- of the multigene family because they create linkage ranged in small tandem clusters on five pairs of chro- disequilibrium among different members of familythe mosomes (for reviews, see LONG and DAWID1980; (OHTA1980a, b;NAGYLAKI and PETES1982; SLATKIN WILSON1982). -

The Effects of Natural Selection on Linkage Disequilibrium and Relative Fitness in Experimental Populations of Drosophila Melanogaster
THE EFFECTS OF NATURAL SELECTION ON LINKAGE DISEQUILIBRIUM AND RELATIVE FITNESS IN EXPERIMENTAL POPULATIONS OF DROSOPHILA MELANOGASTER GRACE BERT CANNON] Department of Zoology, Washington University, St. Louis, Missouri Received April 16, 1963 process of natural selection may be studied in laboratory populations in '?to ways. First, the genetic changes which occur during the course of micro- evolutionary change can be followed and, second, accompanying this, the size of the populations can be measured. CARSON(1961 ) considers the relative size of a population to be an important measure of relative population fitness when com- paring genetically different populations of the same species under uniform en- vironmental conditions over a period of time. In the present experiment, the experimental procedure of CARSONwas utilized to study the effects of selection on certain gene combinations and to measure the level of relative population fitness reached during the microevolutionary process. Experimental populations were constructed with certain oligogenes in low fre- quency and in certain associations in order to provide a situation likely to be changed by natural selection. Specifically, oligogenes on the third chromosome were allowed to recombine freely with the homologous Oregon chromosome so that three separate blocks could be selected for introduction into homozygous Oregon populations. The introduction contained all five of the oligogenes. At intervals samples were re- moved from the populations and testcrossed to determine whether selection had favored the coupling or repulsion phases of these blocks. In addition, the fitness of the populations was measured. This paper will show first the changes in frequency of the various gene combi- nations which occurred in the three experimental populations. -

Fisher's Fundamental Theorem of Natural Selection Steven A
TREE vol. 7, no. 3, March 1992 Fisher's Fundamental Theorem of Natural Selection Steven A. Frank and Montgomery Slatkin relationship to Adaptive Land- scapes. We focus on three ques- tions: What did Fisher really mean by the Fundamental Theorem? is Fishes Fundamental Theorem of natural though it were governed by the Fisher's interpretation of the Fun- selection is one of the most widely cited average condition of the species or damental Theorem useful? Why was theories in evolutionary biology. Yet it has inter-breeding group' (Ref. 3, p. 58). Fisher misinterpreted, even though been argued that the standard interpret- Fisher also pointed out that he stated on many occasions that he ation of the theorem is very different from average fitness, measured by the was not talking about the average what Fisher meant to say. What Fisher intrinsic (malthusian) rate of in- fitness of a population? really meant can be illustrated by look- crease of a species, must fluctuate ing in a new way at a recent model for about zero (Ref. 4, pp. 41-45). What did Fisher really mean? the evolution of clutch size. Why Fisher Otherwise, if a species' rate of The standard interpretation of was misunderstood depends, in part, on the increase were consistently positive, the Fundamental Theorem is that contrasting views of evolution promoted by it would soon overrun the world, or natural selection increases the Fisher and Wright. if a species' rate of increase were average fitness of a population at a consistently negative, it would rate equal to the genetic variance in R.A. -

Quantifying Male Attractiveness Nationsbased on Values Andrewards
Received 28October 2002 Accepted 13March 2003 Publishedonline 25 July 2003 Quantifyingmale attractivene ss JohnM. McNamara 1* ,AlasdairI. Houston 2,Miguel Marques dosSantos 1, Hanna Kokko3† and RobBrooks 4 1Departmentof Mathematics, University ofBristol, University Walk,Bristol BS8 1TW, UK 2Schoolof Biological Sciences, University ofBristol, Woodland Road, Bristol BS8 1GU, UK 3Division ofEnvironmentaland EvolutionaryBiology, Institute ofBiomedical and Life Sciences, University ofGlasgow, GlasgowG12 8QQ, UK 4Schoolof Biological Earth and EnvironmentalSciences, TheUniversity ofNew SouthWales, Sydney, NSW 2052,Australia Geneticmodels of sexual selectionare concernedwith adynamic processin which female preferenceand male trait values coevolve.We present a rigorous methodfor characterizing evolutionary endpointsof this processin phenotypic terms.In ourphenotypic characterization themate-choice strategy offemale popu- lation members determineshow attractive femalesshould find each male, anda population is evol- utionarily stable if population members are actually behaving in this way.This provides ajustificationof phenotypic explanations ofsexual selectionand the insights into sexual selectionthat they provide. Fur- thermore, thephenotypic approach also has enormousadvantages over ageneticapproach whencomput- ing evolutionarily stable mate-choicestrategies, especially whenstrategies are allowed tobecomplex time- dependentpreference rules. For simplicity andclarity ouranalysis dealswith haploid mate-choicegenetics anda male trait that is inherited -

Evolution of Female Multiple Mating: a Quantitative Model of the “Sexually Selected Sperm” Hypothesis
ORIGINAL ARTICLE doi:10.1111/evo.12550 Evolution of female multiple mating: A quantitative model of the “sexually selected sperm” hypothesis Greta Bocedi1,2 and Jane M. Reid1 1Institute of Biological and Environmental Sciences, University of Aberdeen, Zoology Building, Tillydrone Avenue, Aberdeen AB24 2TZ, United Kingdom 2E-mail: [email protected] Received June 5, 2014 Accepted October 1, 2014 Explaining the evolution and maintenance of polyandry remains a key challenge in evolutionary ecology. One appealing explana- tion is the sexually selected sperm (SSS) hypothesis, which proposes that polyandry evolves due to indirect selection stemming from positive genetic covariance with male fertilization efficiency, and hence with a male’s success in postcopulatory competition for paternity. However, the SSS hypothesis relies on verbal analogy with “sexy-son” models explaining coevolution of female preferences for male displays, and explicit models that validate the basic SSS principle are surprisingly lacking. We developed analogous genetically explicit individual-based models describing the SSS and “sexy-son” processes. We show that the analogy between the two is only partly valid, such that the genetic correlation arising between polyandry and fertilization efficiency is generally smaller than that arising between preference and display, resulting in less reliable coevolution. Importantly, indirect selection was too weak to cause polyandry to evolve in the presence of negative direct selection. Negatively biased mutations on fertilization efficiency did not generally rescue runaway evolution of polyandry unless realized fertilization was highly skewed toward a single male, and coevolution was even weaker given random mating order effects on fertilization. Our models suggest that the SSS process is, on its own, unlikely to generally explain the evolution of polyandry. -

The Role of Linkage Disequilibrium in the Evolution of Premating Isolation
Heredity (2009) 102, 51–56 & 2009 Macmillan Publishers Limited All rights reserved 0018-067X/09 $32.00 www.nature.com/hdy SHORT REVIEW The role of linkage disequilibrium in the evolution of premating isolation MR Servedio Department of Biology, University of North Carolina, Chapel Hill, NC, USA The suggestion that speciation may often occur, or be such factors: one-allele versus two-allele mechanisms of completed, in the presence of gene flow has long been premating isolation, and the form of selection against hybrids contentious, due to an appreciation of the challenges to as it relates to its effect on the pathway between post- maintaining population- or species-specific gene combina- zygotic and prezygotic isolation. The goal of this discussion tions when gene flow is occurring. Linkage disequilibrium is not to thoroughly review these factors, but instead to between loci involved in postzygotic and premating isolation concentrate on aspects and implications of these solutions must often be built and maintained as the source of these that are currently underemphasized in the speciation species-specific genotypes. Here, I discuss proposed solu- literature. tions to facilitate the establishment and maintenance of Heredity (2009) 102, 51–56; doi:10.1038/hdy.2008.98; this linkage disequilibrium. I concentrate primarily on two published online 24 September 2008 Keywords: gene flow; recombination; reinforcement; speciation; sympatric speciation Introduction on the build-up of linkage disequilibrium between genes involved in premating and postzygotic isolation. Here, I There has been a long-standing emphasis in speciation discuss solutions that have been proposed to ease the research on describing conditions that may facilitate the conditions for speciation with gene flow. -

Ornamentation, Behavior, and Maternal Effects in the Female Northern Cardinal
The University of Southern Mississippi The Aquila Digital Community Master's Theses Summer 8-2011 Ornamentation, Behavior, and Maternal Effects in the Female Northern Cardinal Caitlin Winters University of Southern Mississippi Follow this and additional works at: https://aquila.usm.edu/masters_theses Part of the Biology Commons, and the Ornithology Commons Recommended Citation Winters, Caitlin, "Ornamentation, Behavior, and Maternal Effects in the Female Northern Cardinal" (2011). Master's Theses. 240. https://aquila.usm.edu/masters_theses/240 This Masters Thesis is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Master's Theses by an authorized administrator of The Aquila Digital Community. For more information, please contact [email protected]. The University of Southern Mississippi ORNAMENTATION, BEHAVIOR, AND MATERNAL EFFECTS IN THE FEMALE NORTHERN CARDINAL by Caitlin Winters A Thesis Submitted to the Graduate School of The University of Southern Mississippi in Partial Fulfillment of the Requirements for the Degree of Master of Science Approved: _Jodie M. Jawor_____________________ Director _Frank R. Moore_____________________ _Robert H. Diehl_____________________ _Susan A. Siltanen____________________ Dean of the Graduate School August 2011 ABSTRACT ORNAMENTATION, BEHAVIOR, AND MATERNAL EFFECTS IN THE FEMALE NORTHERN CARDINAL by Caitlin Winters August 2011 This study seeks to understand the relationship between ornamentation, maternal effects, and behavior in the female Northern Cardinal (Cardinalis cardinalis). Female birds possess ornaments that indicate a number of important known aspects of quality and are usually costly to maintain. However, the extent to which female specific traits, such as maternal effects, are indicated is less clear. It is predicted by the Good Parent Hypothesis that this information should be displayed through intraspecific signal communication. -

Evolution of Female Choice Under Intralocus Sexual Conflict and Rstb.Royalsocietypublishing.Org Genotype-By-Environment Interactions
Downloaded from http://rstb.royalsocietypublishing.org/ on August 27, 2018 Evolution of female choice under intralocus sexual conflict and rstb.royalsocietypublishing.org genotype-by-environment interactions Xiang-Yi Li1 and Luke Holman2 1Department of Evolutionary Biology and Environmental Studies, University of Zurich, Winterthurerstrasse 190, Research 8057 Zurich, Switzerland 2School of BioSciences, University of Melbourne, Parkville, Victoria 3010, Australia Cite this article: Li X-Y, Holman L. 2018 Evolution of female choice under intralocus X-YL, 0000-0001-8662-0865 sexual conflict and genotype-by-environment In many species, females are hypothesized to obtain ‘good genes’ for their interactions. Phil. Trans. R. Soc. B 373: offspring by mating with males in good condition. However, female pre- 20170425. ferences might deplete genetic variance and make choice redundant. http://dx.doi.org/10.1098/rstb.2017.0425 Additionally, high-condition males sometimes produce low-fitness offspring, for example because of environmental turnover and gene-by-environment interactions (GEIs) for fitness, or because fit males carry sexually antagonistic Accepted: 24 July 2018 alleles causing them to produce unfit daughters. Here, we extend previous theory by investigating the evolution of female mate choice in a spatially One contribution of 14 to a theme issue explicit evolutionary simulation implementing both GEIs and intralocus ‘Linking local adaptation with the evolution of sexual conflict (IASC), under sex-specific hard or soft selection. We show sex differences’. that IASC can weaken female preferences for high-condition males or even cause a preference for males in low condition, depending on the relative benefits of producing well-adapted sons versus daughters, which in turn Subject Areas: depends on the relative hardness of selection on males and females. -

The Effect of Purging on Sexually Selected Traits Through Antagonistic Pleiotropy with Survival Geir H
The effect of purging on sexually selected traits through antagonistic pleiotropy with survival Geir H. Bolstad1, Christophe Pelabon´ 1, Line-K. Larsen2, Ian A. Fleming3, Aslaug˚ Viken2 & Gunilla Rosenqvist1 1Centre for Conservation Biology, Department of Biology, Norwegian University of Science and Technology, 7491 Trondheim, Norway 2Norwegian Biodiversity Information Centre, Erling Skakkes gt. 47, 7491 Trondheim, Norway 3Ocean Sciences Centre, Memorial University of Newfoundland, St. John’s, Newfoundland, Canada A1C 5S7 Keywords Abstract Contextual analysis, genetic architecture, genetic correlation, Poecilia reticulata, Sexuallyselectedtraitsareexpectedtoevolvetoapointwheretheirpositiveeffect sexually selected traits. on reproductive success is counterbalanced by their negative effect on survival. At the genetic level, such a trade-off implies antagonistic pleiotropy between sur- Correspondence vival and the expression of sexually selected traits. Yet, the consequences of such Geir H. Bolstad, Centre for Conservation a genetic architecture have been largely overlooked in studies examining how in- Biology, Department of Biology, Norwegian breeding influences sexually selected traits. These studies have solely interpreted University of Science and Technology, 7491 Trondheim, Norway. Tel: +47 92 03 76 their results as an effect of increased homozygosity. An alternative, however, is 65; Fax: +47 735 96100; that purging of recessive alleles deleterious for survival when inbreeding increases E-mail: [email protected] can negatively affect the expression of sexually selected traits through antagonistic pleiotropy. We tested this hypothesis by analyzing the effects of inbreeding on sev- Received: 15 November 2011; Revised: 24 eral male ornaments and life-history traits across 20 captive populations of guppies February 2012; Accepted: 24 February 2012 (Poecilia reticulata) with varying levels of inbreeding. Only one ornament, orange area, decreased in its expression with an increasing level of inbreeding. -

Linkage Disequilibrium and Its Expectation in Human Populations
Linkage Disequilibrium and Its Expectation in Human Populations John A. Sved School of Biological Sciences, University of Sydney, Australia inkage disequilibrium (LD), the association in popu- By the time that the possibility of LD mapping was Llations between genes at linked loci, has achieved realized (e.g., Ikonen, 1990) the terminology of LD a high degree of prominence in recent years, primar- was well established. Currently the term is increas- ily because of its use in identifying and cloning genes ingly used, with many thousands of PubMed of medical importance. The field has recently been references and over 100 Wikipedia references. Note reviewed by Slatkin (2008). The present article is also the confusion with ‘lethal dose’, ‘learning disabili- largely devoted to a review of the theory of LD in ties’ and other terms in literature searches involving populations, including historical aspects. the LD acronym. The chance of any more appropriate Keywords: linkage disequilibrium, linked identity-by- terminology seems to have passed, even if a simple descent, LD mapping, composite D, Hapmap and suitable term could be suggested. ‘Allelic associa- tion’ would seem a desirable term (e.g., Morton et al., 2001) but for the fact that the genes involved are, by definition, non-allelic. Other authors have preferred to The Terminology of LD use the term ‘gametic phase disequilibrium’ (e.g., It has been clear for many years that LD is prevalent Falconer & Mackay, 1996; Denniston, 2000) to take across much of the human genome (e.g., Conrad account of the possibility of LD between genes on dif- 2006), and presumably any other organism when it is ferent, a situation that can arise when many unlinked looked for (e.g., Farnir et al., 2000, in cattle). -
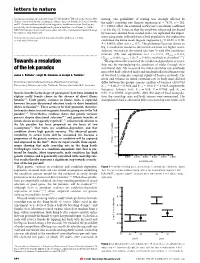
Towards a Resolution of the Lek Paradox
letters to nature discussions regarding experimental design. We also thank G. Taylor from the Oregon State mating. The probability of mating was strongly affected by Climate Center for help with obtaining the climate data. J. A. Pounds, E. Post, M. Vronsky the male's courtship rate (logistic regression x2 79:76, n 232, and N. Chevoteravich provided helpful suggestions on this manuscript. Funding was 1 provided by the Declining Amphibian Population Task Force Seed Grant (to J.M.K.); P , 0.0001; effect size estimated as Pearson's correlation coef®cient NIH/NSF Ecology of Infectious Diseases grant (to J.M.K.); and the Department of Biology, r 0:6; Fig. 1). To ensure that this result was robust and not biased Pennsylvania State University. by measures derived from related males, we replicated the experi- Correspondence and requests for materials should be addressed to J.M.K. ment using males collected from a ®eld population: this replication 2 (e-mail: [email protected]). con®rmed the above result (logistic regression x1 43:30, n 80, P , 0.0001; effect size r 0:7). The preference function shown in Fig. 1 resulted in moderate directional selection for higher court- ship rate (intensity of directional selection (i) with 95% con®dence ................................................................. intervals (CI) and signi®cance test: i 0:331, CIlower 0:121, 22,23 CIupper 0:491; t400 3:20, P 0:0015; methods as described ). Towards a resolution We experimentally examined the condition-dependence of court- ship rate by manipulating the condition of males through their of the lek paradox nutritional state. -

The Evolution of Sexual Dimorphism: Understanding Mechanisms of Sexual Shape Differences
Chapter 1 The Evolution of Sexual Dimorphism: Understanding Mechanisms of Sexual Shape Differences Chelsea M. Berns Additional information is available at the end of the chapter http://dx.doi.org/10.5772/55154 1. Introduction Understanding the origin of biodiversity has been a major focus in evolutionary and ecological biology for well over a century and several patterns and mechanisms have been proposed to explain this diversity. Particularly intriguing is the pattern of sexual dimorphism, in which males and females of the same species differ in some trait. Sexual dimorphism (SD) is a pattern that is seen throughout the animal kingdom and is exhibited in a myriad of ways. For example, differences between the sexes in coloration are common in many organisms [1] ranging from poeciliid fishes [2] to dragon flies [3] to eclectus parrots (see Figure 1). A B Figure 1. A) Male Eclectus (© Stijn De Win/Birding2asia) B) Female Eclectus (© James Eaton/Birdtour Asia) © 2013 Berns, licensee InTech. This is an open access chapter distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. 2 Sexual Dimorphism Sexual dimorphism is also exhibited in ornamentation, such as the horns of dung beetles [4], the antlers of cervids [5], and the tail of peacocks [6]. Many species also exhibit sexual differences in foraging behavior such as the Russian agamid lizard [7], and parental behavior and territoriality can be dimorphic in species such as hummingbirds [8, 9].