Adirondack Lake Assessment Program 2010
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Final Report Template
Final Repport NEIWPCC Job Code: 0100-306-010 Project Code: L-2015-033 Prepared by: Guy Middleton Lake Manager Upper Saranac Foundation Date Submitted: January 2016 Date Approved: March 2016 DateUpper submitted Saranac Lake Watershed Stewardship ished by Program to Prevent the Spread of Aquatic Invasive Species Contact Information ────────────────────────────────────────────────────────────────── Guy Middleton, Lake Manager Upper Saranac Lake Foundation P.O. Box 564, Saranac Lake, NY 12983 Phone: (518) 796-1052 [email protected] This is a Lake Champlain Basin Program funded project 54 West Shore Road Grand Isle, VT 05458 802.372.3213 www.lcbp.org Upper Saranac Lake Watershed Stewardship Program to Prevent the Spread of Aquatic Invasive Species This project was funded by an agreement awarded by the [Environmental Protection Agency/Great Lakes Fishery Commission] to the New England Interstate Water Pollution Control Commission in partnership with the Lake Champlain Basin Program. NEIWPCC manages LCBP’s personnel, contract, grant, and budget tasks and provides input on the program’s activities through a partnership with the LCBP Steering Committee. Although the information in this document has been funded wholly or in part by the United States Environmental Protection agency under agreement CE982720010 to NEIWPCC, it has not undergone the Agency’s publications review process and therefore, may not necessarily reflect the views of the Agency and no official endorsement should be inferred. The viewpoints expressed here do not necessarily represent those of NEIWPCC, the LCBP Steering Committee, or EPA, nor does mention of trade names, commercial products, or causes constitute endorsement or recommendation for use. Table of Contents Page Executive Summary................................................................................... -

Invasive Aquatic Species with the Potential to Affect the Great Sacandaga Lake Region
Invasive Aquatic Species with the Potential to Affect the Great Sacandaga Lake Region Tiffini M. Burlingame, Research Associate Lawrence W. Eichler, Research Scientist Charles W. Boylen, Associate Director Darrin Fresh Water Institute 5060 Lakeshore Drive Bolton Landing, NY 12814 TABLE OF CONTENTS FISH Alewife (Alosa pseudoharengu)s 3 Goldfish (Carassius auratus) 5 Northern Snakehead (Channa argus) 7 Grass Carp (Ctenopharyngodon idella) 9 Eurasian Ruff (Gymnocephalus cernuus) 11 Brook Silverside (Labidesthes sicculus) 13 White Perch (Morone americana) 15 Round Goby (Neogobius melanostomus) 17 Rainbow Trout (Oncorhynchus mykiss) 19 Sea Lamprey (Petromyzon marinus) 21 White Crappie (Pomoxis annularis) 23 Tubenose Goby (Proterorhinus marmoratus) 25 Brown Trout (Salmo trutta) 27 European Rudd (Scardinius erythrophthalmus) 29 Tench (Tinca tinca) 31 PLANTS & ALGAE Ribbon Leaf Water Plantain (Alisma gramineum) 34 Flowering Rush (Butomus umbellatus) 36 Fanwort (Cabomba caroliniana) 38 Rock Snot (Didymosphenia geminata) 40 Brazilian Elodea (Egeria densa) 42 Water Hyacinth (Eichhornia crassipes) 44 Hydrilla (Hydrilla verticillata) 46 Frogbit (Hydrocharis morsus-ranae) 48 Yellow Flag Iris (Iris pseudacorus) 50 Purple Loosestrife (Lythrum salicaria) 52 Water Clover (Marsilea quadrifolia) 54 Parrot Feather (Myriophyllum aquaticum) 56 Variable Leaf Milfoil (Myriophyllum heterophyllum) 58 Eurasian Water Milfoil (Myriophyllum spicatum) 60 Southern Naiad (Najas guadalupensis) 62 Brittle Naiad (Najas minor) 64 Starry Stonewort (Nitellopsis obtusa) 66 Yellow -

VILLAGE of NORTHVILLE DRAFT COMPREHENSIVE PLAN Village Board Review Rev 1
2017 VILLAGE OF NORTHVILLE DRAFT COMPREHENSIVE PLAN Village Board Review Rev 1 ADOPTED: RESOLUTION: ~ 0 ~ VISION STATEMENT In 2035, as you cross the bridge into Northville you will find the Village to be an age diverse yet community minded population living in a quaint and historical atmosphere having a relaxed lifestyle complimented by a strong commercial tax base. The Village's commercial core area will provide residents and visitors with a wide variety of essential goods and services such as grocery, convenience and hardware stores, pharmacy and a bank. The commercial core will 4also be home to a mix of residential uses located above existing commercial buildings providing for an increase in population density to support the commercial establishments. Excellent emergency services to include ambulance, fire and police will continue to serve and be supported by the local population. A healthcare facility will continue to operate within the Village and continue to provide general healthcare services using the latest technologies. Among these essential service establishments, you will find just as many unique family owned and operated businesses such as restaurants, a recreational outfitter, outdoor sporting goods establishment and specialty foods. The Village will feature a low property tax rate and a government that is proactive, ready to assist and support, financially and by other means initiatives important to its citizens. Housing choices will be available for both senior citizens, millennials and young families alike. The Village shall continue to support and be served by a strong school system educating students for both college and the 21st Century workforce through increased educational technologies and disciplines. -

Vail Mills Development Area
The Vail Mills Development Area offers premier opportunities for infill development in the Hamlet of Vail Mills located at the intersection of NYS Routes 29 and 30; a key gateway to the Great Sacandaga Lake and the Adirondack State Park. Fulton County - A Posi+ive Place to Be. VAIL MILLS DEVELOPMENT AREA Enhancing and Growing a Vibrant Hamlet TOWN OF MAYFIELD FULTON COUNTY NEW YORK VAIL MILLS DEVELOPMENT AREA The Vail Mills COMMERCIAL 9 Development Area Vail Mills Development Area Concept Plan ADIRONDACK DEVELOPMENT TRAIL Town of Mayfield, Fulton County, New York ANIMAL LAND AREA CONNECTION TO offers premier BROADALBIN opportunities for KENNYETTO infill development in CREEK the Hamlet of Vail CANOE FJ&G RAIL RESTAURANT LAUNCH Mills located at the TRAIL SITE EXPANSION PROPOSED intersection of NYS PUB/ PARK Routes 29 and 30; a RESTAURANT key gateway to the 6 FJ&G RAIL 2 HOTEL SITE TRAIL Great Sacandaga 5 COMMERCIAL Lake and the 7 4 DEVELOPMENT Adirondack State WITH DESIGNATED Park. Vail Mills is 1 10 ROADWAY 8 adjacent to the 3 11 EXISTING historic Village of RESIDENTIAL Broadalbin in scenic 12 DEVELOPMENT Fulton County, NY. 13 14 As a “Gateway to the Adirondacks,” DAYCARE the Vail Mills Development Area 15 16 RESIDENTIAL is witness to over ADULT/ DEVELOPMENT RESIDENTIAL 10,000 average daily OF 1/2 AND 1 SENIOR HOUSING DEVELOPMENT traffic volumes. The ACRE LOTS PHARMACY/ OF 1/2 AND 1 17 development area RETAIL ACRE LOTS includes several FARMERS MARKET LAUNDROMAT successful businesses VAIL MILLS OCCUPIED PARCELS and a rail trail, a PARCEL PARCEL NAME RETAIL proposed park and NUMBER ADULT/ 1 PETOFF GARDEN APARTMENTS SENIOR TAKE OUT a canoe launch at 2 RESIDENTIAL NEIGHBORHOOD HOUSING RESTAURANT 3 DUNKIN’ DONUTS BANK Kennyetto Creek. -

2017 New York Loon Census Results
ADIRONDACK CENTER FOR LOON CONSERVATION 2017 NEW YORK LOON CENSUS RESULTS On July 15, 2017, more than 450 observers participated in the 17th Annual New York Loon Census, observing a total of 633 loons on 155 (81%) of the 192 New York Loon Census lakes, including 544 (86%) adult, 81 (13%) chick, and 8 (1%) immature loons. “This is the first year since we have been on Garnet Lake (30+ years) that we have had 2 successful nesting and hatchings! Each family has 1 surviving chick. We are able to count 4 visiting loons enjoying the lake today.” ~Garnet Lake “They seemed very relaxed - hooting to the kids. Those are some big healthy looking chicks! Also there must have been a lot of fish - they just skimmed their bills in the water to come up with a fish. I don't think I've ever seen that before.” ~Jones Pond Adirondack Center for Loon Conservation 15 Broadway, Saranac Lake, NY 12983 Mailing: P.O. Box 195, Ray Brook, NY 12977 518-354-8636 [email protected] www.adkloon.org www.facebook.com/adkloon Of the 256 Census reports received in 2017, 253 were included in the analysis, as three reports had observations that were conducted outside the Census hour or were duplicated efforts on a lake. “Population in our area is increasing. Observed a group of 7 adults earlier in spring. As we were observing the second pair this morning, they both began the tremolo call. A minute later a bald eagle flew directly over with an eye on the loons.” ~St. -

OPERATION of GREAT SACANDAGA LAKE – Q & A
OPERATION of GREAT SACANDAGA LAKE – Q & A Q. What rules govern how the Great Sacandaga Lake is operated? Specifically how much water is let out, and when? A. An agreement between interested parties and stakeholder organizations was reached in 2000 and became part of a Federal Energy Regulatory Commission (FERC) license for the Great Sacandaga Lake in 2002. This agreement is known as the “Offer of Settlement” and governs how much water is to be released each day for all combinations of reservoir elevation and downstream flows. Q. What prompted the need for this agreement? A. A relicensing of the hydroelectric power plant at the Conklingville Dam with FERC required the development of an operating plan with appropriate long-term environmental protection measures that would meet diverse objectives for maintaining a balance of interests in the Upper Hudson River Basin. Q. What groups signed on to the agreement? A. A total of 29 organizations approved the agreement. In addition to the Hudson River – Black River Regulating District, they were: Great Sacandaga Lake Association, Fulton County Board of Supervisors, Saratoga County Board of Supervisors, Town of Hadley, Glens Falls Feeder Alliance, Adirondack Boardsailing Club, Adirondack Council, Great Sacandaga Fisheries Association, Erie Boulevard Hydropower, Niagara Mohawk Power Corporation, Adirondack Mountain Club, Glens Falls Chapter of Adirondack Mountain Club, Great Sacandaga Lake Marinas, Adirondack Park Agency, Adirondack River Outfitters, American Rivers, Hudson River Rafting Company, International Paper, American Whitewater, NYS Department of Environmental Conservation, NYS Conservation Council, National Park Service, U.S. Fish and Wildlife Service, Association for the Protection of the Adirondacks, Sacandaga Outdoor Center, Wild Waters Outdoor Center, and New York Council of Trout Unlimited. -

Factual Background
Case 5:09-cv-00471-NAM-ATB Document 63 Filed 05/06/10 Page 1 of 6 UNITED STATES DISTRICT COURT NORTHERN DISTRICT OF NEW YORK ____________________________________________ NIAGARA MOHAWK POWER CORPORATION, d/b/a NATIONAL GRID, Plaintiff, vs. 5:09-CV-00471 HUDSON RIVER-BLACK RIVER REGULATING DISTRICT Defendant. ____________________________________________ NAM APPEARANCES: OF COUNSEL: Hiscock & Barclay, L.L.P. Mark D. Lansing, Esq. 50 Beaver Street Albany, New York 12207-2830 Attorneys for Plaintiff Crane, Parente & Cherubin David M. Cherubin, Esq. Regulating District 90 State Street, Suite 1515 Albany, New York 12207 Attorneys for Defendant Hudson River-Black River Regulating District Hodgson Russ LLP Daniel A. Spitzer, Esq. The Guaranty Building Benjamin K. Ahlstrom, Esq. 140 Pearl Street, Suite 100 Buffalo, New York 14202-4040 Attorneys for Proposed Intervenors Sacandaga Protection Corporation Norman A. Mordue, Chief U.S. District Judge: MEMORANDUM DECISION AND ORDER I. INTRODUCTION This Court previously denied plaintiff’s motion for a preliminary injunction and dismissed the New York State Department of Environmental Conservation (“DEC”) as a defendant. Familiarity with the previous memorandum and decision is assumed. Presently before the Court is a motion to intervene as of right pursuant to Fed. R. Civ. P. 24 (a)(2) by the Sacandaga Case 5:09-cv-00471-NAM-ATB Document 63 Filed 05/06/10 Page 2 of 6 Protection Corporation (“SPC”) as well as a motion for summary judgment by defendant Hudson River-Black River Regulating District (“the District”). For obvious reasons, the Court will address SPC’s motion to intervene first and, if necessary, provide said party an opportunity to submit papers in support of, or in opposition to the pending motion for summary judgment. -

2015 Saratoga Lake Steward Report
ADIRONDACK WATERSHED INSTITUTE SARATOGA LAKE 2015 LOCATION USE SUMMARY 160 STEWARDSHIP PROGRAM Saratoga Lake Boats inspected: 5,811 Visitors taking spread prevention measures: 69% AIS intercepted: 759 Inspected boats with organisms: 18.8% Number of visitors: 13,402 Number of previously visited waterways: 53 Boat Type total # Waterbody M PWC S C K B R SUP Docks boats Saratoga Lake 5422 262 24 24 150 0 15 8 0 5905 percentage of total boats 92% 4% 0% 0% 3% 0% 0% 0% 0% 100% Boats observed at launch. M = motorboat; PWC = personal watercraft; S = sailboat; C = canoe; K = kayak; B = construction barge; R = rowboat; SUP= stand-up paddleboard; Docks = boat docks launched for seasonal installation/maintenance total # organisms found Waterbody # boats # of % of inspected people entering leaving dirty inspections boats dirty Saratoga Lake 13402 649 1310 1092 5811 18.8% boats dirty = watercraft with any organic material, invasive, non-invasive or unknown. # groups taking AIS spread prevention measures Waterbody # groups yes I WB DB BB LW Dis Dry didn't ask asked Saratoga Lake 3186 1162 2381 694 43 155 16 538 1148 4625 percentage of total # groups asked 69% 25% 51% 15% 1% 3% 0% 12% NA Yes = took one or more AIS spread prevention measures; I = inspected boat; WB = washed boat; DB = drained bilge; BB = emptied bait bucket; LW = drained livewell; Dis = disposed of unused bait; Dry = dried boat. Organism Type % of inspected Waterbody total BW CLP* ELO GRS EWM* NM UM VLM* MUD NON NP SWF* WC* WL ZM* other AIS boats with AIS Saratoga Lake 2 189 172 664 356 60 0 0 10 36 233 0 28 5 186 18 759 13.1% percentage of organisms removed 0% 10% 9% 34% 18% 3% 0% 0% 1% 2% 12% 0% 1% 0% 9% 1% BW = bladderwort; CLP = curly-leaf pondweed; ELO = elodea; GRS = grass; EWM = Eurasian watermilfoil; NM = native milfoil; UM = unknown milfoil; VLM = variable leaf milfoil; MUD = mud; NON = non-aquatic debris; NP = native pondweed; SWF = spiny waterflea; WC= water chestnut; WL= water lily; ZM = Zebra mussel; */AIS = aquatic invasive species. -

Freshwater Fishing Regulation Guide
NEW YORK STATE FRESHWATER FISHING REGULATIONS GUIDE Regulations in efect April 1, 2021 Department of Environmental Conservation New York State has about 300 Complaints are forwarded to an ECO Environmental Conservation Ofcers for investigation. The more detailed (ECOs) and Investigators (ECIs) who work information you provide, the more likely throughout the state, including New the violator will be apprehended. Try to York City. ECOs spend most of their time remember the “who, what, where, when, patrolling within their assigned county. and how" of the event. The assistance of the public is essential • Keep a distance from the violator. Do to the efective enforcement of state not approach or attempt to confront environmental laws and regulations. If you suspects. They may be dangerous, observe someone violating Environmental destroy evidence, or simply evade Conservation Law or see the results of ofcers if forewarned. a violation, REPORT IT! Poachers and • Who did it? Provide names, ages, sex, polluters are thieves, stealing from you, height, weight, clothing or vehicle our fellow anglers, and future generations. descriptions, and other details. Those who pollute our air or water, destroy • What occurred? What exactly do you our environment, or ignore fsh and wildlife think is the nature of the violation? laws are criminals. Examples — taking over limit of fsh, Contact an Environmental snagging, illegal netting, fshing out of season, trespassing. Conservation Police Ofcer • When did it occur? Provide dates and (ECO) times. Is it still in progress, ongoing, or For general questions, call 1-877-457-5680. something yet to happen? Examples— You will speak with a dispatcher who will happening right now, happens every Fri assist you or connect you to an ECO. -
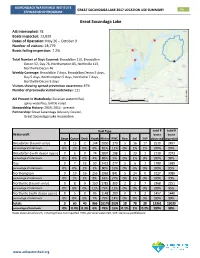
Great Sacandaga Lake 2017 Location Use Summary 96 Stewardship Program
ADIRONDACK WATERSHED INSTITUTE GREAT SACANDAGA LAKE 2017 LOCATION USE SUMMARY 96 STEWARDSHIP PROGRAM Great Sacandaga Lake AIS intercepted: 78 Boats inspected: 13,039 Dates of Operation: May 26 – October 9 Number of visitors: 28,779 Boats failing inspection: 7.2% Total Number of Days Covered: Broadalbin 110, Broadalbin Decon 52, Day 76, Northampton 85, Northville 115, Northville Decon 46 Weekly Coverage: Broadalbin 7 days, Broadalbin Decon 5 days, Day 5 days, Northampton 5 days, Northville 7 days, Northville Decon 5 days Visitors showing spread prevention awareness: 87% Number of previously visited waterways: 111 AIS Present in Waterbody: Eurasian watermilfoil, spiny waterflea, brittle naiad Stewardship History: 2009, 2014 - present Partnership: Great Sacandaga Advisory Council, Great Sacandaga Lake Association Boat Type total # total # Watercraft boats boats Barge Canoe Dock Kayak Motor PWC Row Sail SUP observed inspected Broadalbin (launch only) 0 15 2 144 2050 270 5 16 17 2519 2497 percentage of total boats 0% 1% 0% 6% 81% 11% 0% 1% 1% 100% 99% Broadalbin (with decon open) 0 6 0 74 1807 198 1 23 1 2110 2073 percentage of total boats 0% 0% 0% 4% 86% 9% 0% 1% 0% 100% 98% Day 0 7 13 50 1421 277 5 6 1 1780 1689 percentage of total boats 0% 0% 1% 3% 80% 16% 0% 0% 0% 100% 95% Northampton 0 19 16 253 1969 841 5 24 0 3127 3089 percentage of total boats 0% 1% 1% 8% 63% 27% 0% 1% 0% 100% 99% Northville (launch only) 0 8 9 250 1781 309 2 2 7 2368 2251 percentage of total boats 0% 0% 0% 11% 75% 13% 0% 0% 0% 100% 95% Northville (with decon open) 0 5 0 95 1148 200 0 7 2 1457 1440 percentage of total boats 0% 0% 0% 7% 79% 14% 0% 0% 0% 100% 99% totals 0 60 40 866 10176 2095 18 78 28 13361 13039 percentage of total boats 0% 0.4% 0.3% 6% 76% 16% 0.1% 1% 0.2% 100% 98% Boats observed at launch, including those not inspected. -

90Th Anniversary of the Creation of the Great Sacandaga Lake Lauren Roberts, Saratoga County Historian
90th Anniversary of the Creation of the Great Sacandaga Lake Lauren Roberts, Saratoga County Historian Ninety years ago today life in the Sacandaga Valley changed forever. March 27, 1930 marked the completion of the Conklingville Dam and the closing of its three large Dow valves, which hold back the 37 billion cubic feet of water we now know as the Great Sacandaga Lake. While the day was marked with a small gathering, there was no elaborate ceremony. As all upstate New Yorkers know too well, the weather in March can be problematic for outdoor gatherings. The pomp and circumstance of long speeches from honorary guests eventually occurred in September of that year, when the weather was much more pleasant. The massive project to build the Sacandaga Reservoir actually began three years prior to this date. Despite local opposition, New York State created the Hudson River Regulating District, giving the new agency the authority to create reservoirs within the Upper Hudson River Watershed in order to impound water, preventing flooding. In addition, water could be released in times of drought. The Sacandaga Reservoir Project would create the largest reservoir in New York State. Ten communities in the valley were either partially or completely flooded. All residents living below “the taking line” needed to move to higher ground. All of the buildings and structures had to be removed, ten new bridges built, miles of new highway constructed. Two additional dams besides Conklingville had to be completed. This massive undertaking cost 12 million dollars, 95% of which was paid for by private companies along the Hudson who would benefit from a guaranteed water supply. -

State of New York
STATE OF NEW YORK Hudson River – Black River Regulating District Contact Information Sacandaga Field Office: 737 Bunker Hill Road Due Process Procedures for Mayfield, New York 12117 Access Permit Holders at Phone: (518) 661-5535 Great Sacandaga Lake Fax: (518) 661-5720 [email protected] Albany Office: 350 Northern Boulevard Albany, New York 12204 Phone: (518) 465-3491 Adopted by Board Resolution Fax: (518) 432-2485 10-04-01 [email protected] January 2010 Great Sacandaga Lake The Board of the Hudson River - determination in a written provide to the Executive Black River Regulating District has response delivered to the access Director nine (9) copies of any adopted a uniform due process permit holder. written materials and/or photos procedure for aggrieved access he or she plans to present to the permit holders at Great Sacandaga Board. The Executive Director Lake who seek to appeal a 3. Second right of appeal to shall, no later than ten (10) days determination made by the District Executive Director. before the hearing, present to Management of the Regulating the Board and the access permit District regarding its administration Executive Director shall holder copies of the information of rules, regulations, and policies. conduct a resolution conference the District plans to submit to with access permit holder(s) the Board. At such hearing the These due process procedures and/or their designee. The Executive Director or his/her apply solely to aggrieved access District shall keep a hearing designee, who shall represent permit holders at Great Sacandaga record. Executive Director shall the Board, and the aggrieved Lake.